-
PDF
- Split View
-
Views
-
Cite
Cite
Ibrahim Afifi, Husham Abdelrahman, Ahmed El-Faramawy, Ismail Mahmood, Sherwan Khoschnau, Noof Al-Naimi, Ayman El-Menyar, Hassan Al-Thani, Sandro Rizoli, The use of Indocyanine green fluorescent in patients with abdominal trauma for better intraoperative decision-making and less bowel anastomosis leak: case series, Journal of Surgical Case Reports, Volume 2021, Issue 6, June 2021, rjab235, https://doi.org/10.1093/jscr/rjab235
Close - Share Icon Share
Abstract
Despite technological advances in the management of blunt abdominal trauma, the rate of bowel anastomotic leakage (AL) remains high. The etiology of AL is multifactorial, but insufficient blood perfusion is considered to play a substantial role in the pathogenesis. In recent years, angiography with Indocyanine green (ICG), a fluorescent dye, has been introduced in the clinical practice to assess organ perfusion in several conditions. Given the scarcity of publications describing the use of ICG in trauma patients as a potentially useful strategy that may facilitate intraoperative decisions and limit the extent of bowel resection, we presented the utility of intraoperative ICG fluorescent in abdominal trauma patients in a level 1 trauma center. The use of ICG fluoroscopy in patients with abdominal trauma is feasible and useful; however, large prospective studies in trauma patients are warranted.
INTRODUCTION
Despite technological advances and improved surgical skills, the incidence of bowel anastomotic leakage (AL) following gastrointestinal surgery ranges between 3 and 20% with a mortality rate of 10–15% [1–3]. AL represents one of the most dreadful complications in general surgery and is associated with significant morbidity and mortality. The etiology of AL is multifactorial, but insufficient blood perfusion is considered to play a substantial role in the pathogenesis [3, 4].
In recent years, angiography with Indocyanine green (ICG), a fluorescent dye, has been introduced in clinical practice to evaluate organ perfusion in several conditions [5, 6].
The blunt trauma patient with bowel injury is at great risk of ischemia at the microcirculation level, which can compromise the integrity of the anastomosis as reported by many.
Also, the extent of the resection and the perfusion of the anastomotic edges are frequently challenging to establish, particularly during damage control laparotomy (DCL). Often temporary abdominal closure and second-look laparotomy are used to determine perfusion of resected margins, thus delaying the anastomosis, and increasing the risk of anastomotic leak.
Furthermore, in challenging cases, the surgeon may opt to include in the resection portion of the bowel that are well perfused to reduce the chances of the anastomotic leak. This would be affecting the length of the remaining bowel and leading the patient at risk of short bowel syndrome or nutritional deficiencies. Considering the scarcity of publications describing the use of this potentially useful strategy that may facilitate intraoperative decisions and limit the extent of bowel resection in trauma, we reviewed the experience of a single level 1 trauma center in using the ICG fluorescent in patients with abdominal injury.
We retrospectively reviewed the data for patients who were admitted in June 2020 when the ICG fluorescent imaging was adopted. The motive to institute this strategy is to reduce the number of AL, reduce the extent of bowel resection and increase the chances to perform a safe bowel anastomosis during the initial operation, thus reducing the need for second-look surgery.
We implement this technique in three cases that underwent DCL with bowel resection without anastomosis. ICG fluoroscopy was used at the follow-up surgery, to assess the vascularity of the bowel before and after anastomosis.
The ICG is available in powder form. The package includes sterile water (for injection) and the green powder (lyophilized 25 mg of ICG with no > 5% sodium iodide). ICG angiography was performed with a fluorescence imaging system (S™, Karl Storz, Germany). ICG (25 mg, Daiichi Sankyo, Tokyo, JP) was diluted in 10 ml of distilled water, and a minimum dose of 0.25 mg/kg was slowly injected into the peripheral blood vessels for 10 s. Intravenous ICG rapidly bound to the intravascular protein (albumin is the principal carrier; 95%) and thus remained in the vascular circulation. Then, a near-infrared ray with a wavelength of 800 nm will be emitted from the laparoscopic camera, causing ICG in the blood vessel to emit a wavelength of 803 nm, and the fluorescence image will be followed on the monitor. The video image can appear in blue or green mode indicating the tissue perfusion. Blood perfusion will be observed for 2 min after ICG injection, if enough blood flow was not observed within 2 min, will wait for another 3 min and the bowel will be observed for up to 5 min. [7]
Diluted ICG is injected using two boluses of 3 ml, each at a concentration of 0.2 mg/kg. The first bolus is administered after mesenteric division facilitating resection by providing relevant information on well-perfused areas. The second bolus is given before bowel anastomosis to confirm adequate vascularization. For extracorporeal bowel division, adequate visualization is feasible only with the operating room lighting turned off, because ambient illumination has been found to interfere with the fluorescence detection sensitivity of the video camera [8].
CASE-1
A 39-year-old male sustained motor vehicle collision (MVC), rollover and ejection. On arrival, he was hypotensive with positive FAST scan, left femur fracture and dislocated right knee. Massive transfusion protocol was activated, and the patient shifted to the theater within minutes of arrival.
The patient underwent DCL where 3 l of intraperitoneal blood with ileal mesenteric laceration and ileal ischemia were identified. Hemostasis was done for mesenteric bleeding by direct ligation of bleeding vessels and resection of 50 cm of distal ilium done with primary anastomosis using stapler technique. The abdomen was temporarily closed with the open abdomen negative pressure therapy system (ABThera) and a second-look laparotomy was planned due to the risk of anastomosis ischemia.
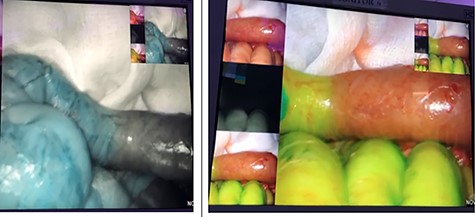
On the second look, we used ICG fluoroscopy technique to confirm anastomosis integrity, one dose of 3 ml was injected and images showed intact anastomosis with full green light identified (Figs 1 and 2). The anastomosis was confirmed for integrity and the abdomen was closed. The patient had an uneventful postoperative course and feeding was resumed on the second postoperative day.
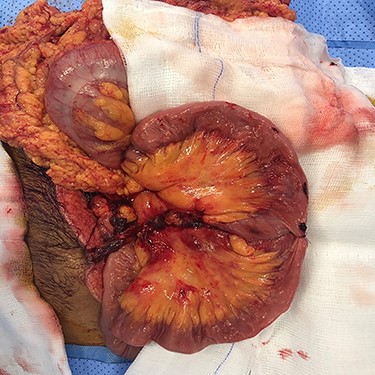
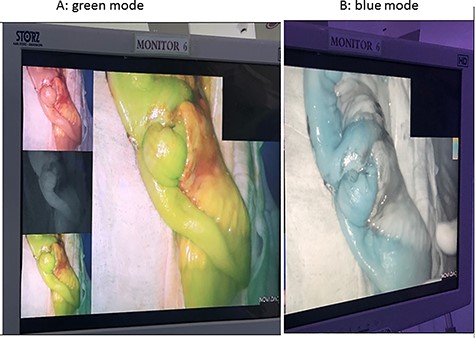
ICG study for bowel ischemia, showed no ischemia with good perfusion.
CASE-2
A 26-year-old male had an assault with multiple stabs to the abdomen with bowel evisceration. The patient was immediately shifted to the theater for exploratory laparotomy. During the surgery, multiple perforations in a short segment of the small bowel and a right colon through and through the perforation were observed. Resection anastomosis of the small bowel and primary repair of the right colon was done, and the abdomen was closed.
The postoperative course was complicated with diarrhea and wound dehiscence with serosanguinous discharge. An abdominal CT scan with triple contrast showed bowel leak from the right colon. The patient was taken for a second laparotomy where a previously unidentified perforation of the colon was recognized with a collection of enteral content in the retroperitoneal space. Right hemicolectomy was performed without anastomosis, the retroperitoneal cavity was drained and the abdomen was temporarily closed with ABThera.
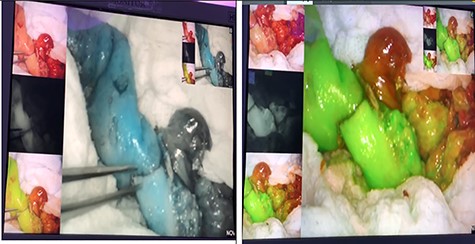
The third laparotomy was done after 48 h, in which ICG technique was used in two doses; the first dose was to show the integrity of the edges and the second dose was given after ileocolic anastomosis to re-assess the perfusion. ICG confirmed the integrity of perfusion and the abdomen was closed with a good postoperative course without evidence of an anastomotic leak (Figs 3 and 4).
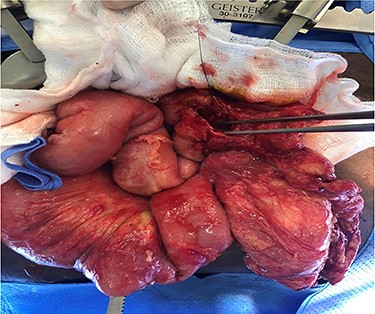
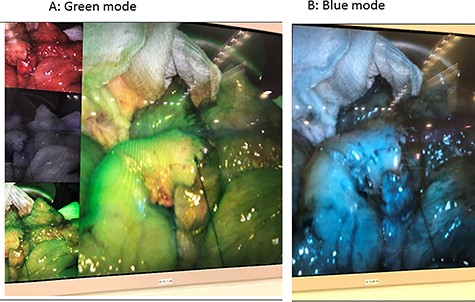
CASE-3
A 46-year-old male victim of MVC presented with hypotension and undetermined FAST scan. The patient responded to initial resuscitation and abdomen CT scan showed mild hemoperitoneum, significant fat stranding and mesenteric hematoma. Exploratory laparotomy revealed avulsed greater omentum from the transverse colon, extensive laceration of right colon mesentery and crushed pancreatic head. Extended right hemicolectomy without anastomosis, partial omentectomy and packing of pancreas head were done and the abdomen left temporarily closed with ABThera technique.
A second-look surgery was performed 48-h later and ICG fluoroscopy was applied which revealed a non-perfused 20 cm of distal ileum (Figs 5–7). Resection of the ischemic segment was done, and the second bolus of ICG was given attesting the good perfusion of the anastomotic edges. This Ileocolic anastomosis was drained along with the pancreatic head while the abdomen was closed. Follow-up magnetic resonance cholangiopancreatography showed no pancreatic ductal injury.
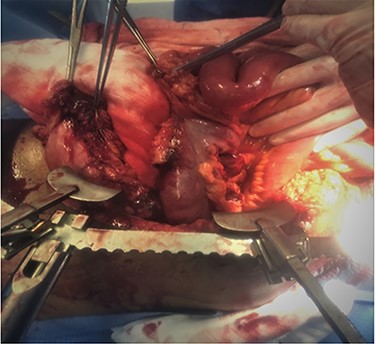
Avulsed greater omentum with large right colon mesenteric laceration and avulsion.
DISCUSSION
Because of its impact on the anastomotic healing, ICG fluorescent was found to be a promising tool in the assessment of tissue (visceral) perfusion [9]. In the present case series, ICG fluorescent was a useful instant tool in assessing and determining bowel perfusion and arguably, preventing anastomotic leak in trauma patients. In the first case, it helped to assess the blood perfusion in a high-risk segment of the bowel with mesenteric lacerations, and thus, helped with the appropriate repair. In the second case, ICG was helpful to assess the viability of the anastomotic edges. In the third case, ICG diagnosed an ischemic 20-cm segment of ileum that appeared macroscopically perfused and hence prevented the creation of an ischemic anastomosis, which would eventually leak.
A recent randomized controlled trial involved 240 patients undergoing laparoscopic left-sided colon and rectal resection randomized 1:1 to intraoperative ICG versus to subjective visual evaluation of the bowel perfusion without ICG. The aim was to explore the use of ICG to decrease the rate of anastomotic leaks, strategy modifications and morbidity. ICG angiography showed insufficient perfusion of the colonic stump, which led to extended bowel resection in 13 cases (11%). An anastomotic leak developed in 11 patients (9%) in the control group and 6 patients (5%) in the study group (not statistically significant) [10].
In a systemic review, 14 studies were included. Ten studies (n = 916) involved patients with colorectal anastomosis. It showed that the use of ICG reduced the risk of bowel leak (3.3% vs. 8.5% for the non-ICG) [11].
In a meta-analysis study, 5 non-randomized studies included 1302 patients, fluorescence imaging significantly reduced the AL rate in patients undergoing surgery for colorectal cancer (OR 0.34; CI 0.16–0.74; P = 0.006). Rectal cancer surgery showed a significantly lower rate of leaks (ICG 1.1% vs. 6.1% for the non-ICG cases). There was no significant decrease in the AL rate when colorectal procedures for benign and malignant diseases were combined [12].
Karampinis et al. [13] in a retrospective analysis of 52 patients with acute mesenteric ischemia who underwent surgery; concluded that the use of ICG fluorescence angiography is a possible and reliable tool for perfusion assessment of the bowel with a rate of clinical benefit of 11%. He also compared gross inspection versus ICG and reported a 35% discrepancy rate, which is relevant in guiding the surgeon in deciding on an accurate margin selection [13].
For intraoperative navigation, surgeons rely, in most cases, solely on the visual and tactile feedback to distinguish between different kinds of tissue structures. Searching for intraoperative tools to facilitate surgical decision-making; the near-infrared (NIR) light spectrum, was tested for different conditions [5–8]. A NIR light (650–900-nm wavelength) is preferred for deeper tissue penetration compared to visible light as it has deep-tissue penetration (up to 10 mm) and good signal-to-background ratio [14]. In this regard, the Food and Drug Administration and the European Medicines Agency approved two contrast agents (ICG and methylene blue [12, 15]).
The ICG dye has no significant tissue uptake in kidneys, lung, CSF or peripheral tissues and enterohepatic and extrahepatic circulation. The only caution for use of the dye is iodide allergy as it contains sodium iodide. Also, radioactive iodine uptake studies should not be performed for at least a week following the use of ICG. The package should be mixed with maximum sterile precaution (25 mg of the dye in 5 ml of water to get a 5 mg/ml concentration; [15]). The ICG dose is variable; the most common is 0.2–0.5 mg/kg. Nevertheless, it was shown that the colorectal anastomotic perfusion could also be visualized by using a much lower and less toxic dose (2.5 mg), which is in concordance with the optimal dose used for evaluation gastroesophageal anastomotic perfusion. Hence, we recommend the lower injection dose of (2.5 mg) for checking anastomosis. The NIR fluorescence imaging should be timed within the 60s of the injection. If the fluorescence signal starts to fade, another bolus of 2.5-mg ICG can be injected to evaluate the perfusion for a second look (>15 min after the first injection). No need for any surgical modification if normal perfusion is demonstrated with NIR imaging [16].
Our previous work in 2020 showed that in 160 trauma patients with bowel injury, 46.3% of patients underwent debridement and primary closure, while 53.8% required resection with anastomosis. The anastomotic leaks in the resection anastomosis group were 13 out of 86 patients (15.1%; [17]).
Although there is a failure rate with ICG fluoroscopy when used with laparoscopic surgical procedures in most articles, in trauma we used it in open technique which adds to the surgical assessment and tactile sensation, thus it hopefully will help in reducing AL in a significant manner.
The use of intraoperative fluorescent angiography was reported in war-related trauma and it was observed that 35 cases (19%) required intraoperative modifications to obtain better tissue perfusion, 9 of the 35 cases had bowel injury (3 required intraoperative modification) [18].
Lastly, it is important to mention the limitation of our small case-series; we have to consider the potential technical failure that could be encountered and the generalized causes and localized factors of bowel AL.
CONCLUSION
The use of ICG fluoroscopy in patients with abdominal trauma is feasible and useful; it leads to extending the resection in one case and ascertaining the anastomosis was created in well-perfused bowel followed by no anastomotic leak in two cases. There are potential benefits for ICG in abdominal trauma undergoing laparotomy to minimize bowel resection in extensive mesenteric laceration with the benefit of avoiding short bowel, and assessment of bowel integrity in mesenteric hematoma to avoid the risk of bowel ischemia. However, large prospective studies on the ICG use in trauma patients are warranted.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
References
Jannasch O, Klinge T, Otto R, Chiapponi C, Udelnow A, Lippert H, et al.
Akorn Inc, Buffalo Grove, IL. https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/011525s017lbl.pdf (22 May 2021, date last accessed).
De Nardi P, Elmore U, Maggi G, Maggiore R, Boni L, Cassinotti E, et al.
van Manen L, Handgraaf HJM, Diana M, Dijkstra J, Ishizawa T, Vahrmeijer AL, et al.



