-
PDF
- Split View
-
Views
-
Cite
Cite
Reid C Mahoney, Kyrillos Awad, Gregorio Maldini, Metastatic neuroendocrine tumor with metastases to the right liver in a patient with absent left portal vein, Journal of Surgical Case Reports, Volume 2021, Issue 5, May 2021, rjab207, https://doi.org/10.1093/jscr/rjab207
Close - Share Icon Share
Abstract
The patient is a 50-year-old female that underwent routine screening colonoscopy during which she was found to have a neuroendocrine tumor in the right colon. The patient underwent computed tomography and magnetic resonance imaging scans that demonstrated metastatic disease in segments 5 and 7 of the liver. Notably, the patient was found to have an absent left portal vein. The metastatic lesions abut the right portal vein; the right portal vein also supplies the left lobe of the liver in place of an absent left portal vein. She underwent a laparoscopic-assisted right hemicolectomy to remove the primary tumor. The patient recovered uneventfully from surgery and is currently being monitored by a multidisciplinary team regarding her metastatic disease. Neuroendocrine tumors can cause long-term effects on health and ultimately death if left untreated. We present a case of metastatic midgut neuroendocrine tumor that has metastasized to the liver in a patient with absent left portal vein.
INTRODUCTION
Gastroenteropancreatic neuroendocrine tumors are divided by the World Health Organization (WHO) as grades 1, 2 or 3 based on mitoses and Ki-67 index [1]. The SEER database demonstrates a non-pancreatic neuroendocrine tumor incidence of 4.7 per 100 000 patients, and recent age-adjusted data is as high as 5.2/100 000 [2]. Separately, portal vein anomalies are rare, and absence of the portal vein bifurcation is even rarer, estimated at 0.3–2% of all portal vein anomalies [3]. We present a case of metastatic neuroendocrine tumor in a patient with an absent left portal vein.
CASE DESCRIPTION
The patient is a 50-year-old female with a history of hypertension, hyperlipidemia and obesity that presented to an outpatient gastroenterologist for routine screening colonoscopy. The patient was found to have a lesion, measured at ~2.5 centimeters in size, near the ileocecal valve. Biopsies were taken at the time of colonoscopy, which demonstrated evidence of a well-differentiated neuroendocrine tumor with invasion into the submucosa. There was no increased mitotic activity (Ki-67 < 3%). The patient was referred to surgery clinic for further evaluation.
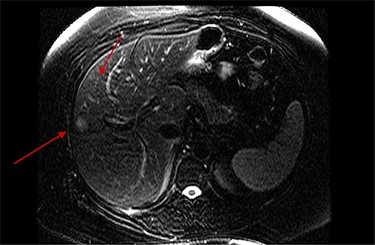
On assessment, the patient denied any symptoms related to her neuroendocrine tumor, including flushing, increased sweating, increased heart rate, wheezing, shortness of breath, diarrhea, weight loss or appetite changes. The only significant family history was a paternal and maternal grandfather with colon cancer. Imaging results were significant for computed tomography (CT) of chest demonstrating very small, but multiple, pulmonary nodules. A CT abdomen and pelvis demonstrated the known neuroendocrine tumor near the ileocecal valve (Fig. 1) as well as two poorly visualized liver lesions (Figs 2–4). The magnetic resonance imaging (MRI) demonstrated two separate one-centimeter lesions in segment 5 and 7 of the liver (Figs 5 and 6). The portal vein lacked normal left and right bifurcation; there was a circumferential right portal vein, which coursed anteriorly and superiorly and ultimately to the left lobe of the liver.
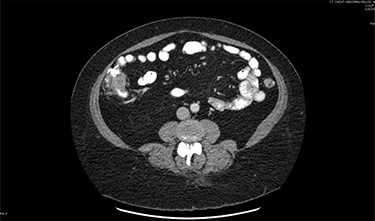
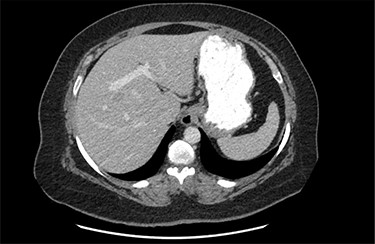
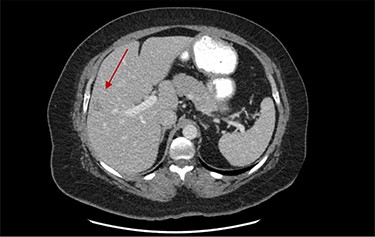
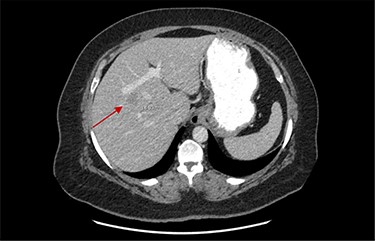
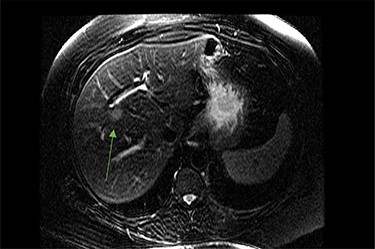
MRI image again demonstrating segment 5 metastatic lesion in close proximity to portal vein.
After workup was completed, the patient was consented for laparoscopic right hemicolectomy for removal of the primary tumor. The procedure was performed in a lateral to medial fashion and a hand-sewn extracorporeal anastomosis was performed after removal of the specimen. The operation was uneventful and the patient recovered appropriately in the immediate postoperative period. She was discharged home tolerating a regular diet and having normal bowel function. Final pathology demonstrated a 3 × 2 × 1.7 cm well-differentiated neuroendocrine tumor. Margins were clear and 8/19 lymph nodes were positive. There were 1/10 mitoses per high power field. Ki-67 was noted to be <1%. The final stage was T3N1M1 (stage IV) by the grading of American Joint Committee on Cancer (AJCC), and the tumor was G1 based on WHO classification.
The patient was seen postoperatively in clinic and has continued to recover well. She underwent repeat imaging that demonstrated stability in the size and location of metastatic disease. She remains asymptomatic. Oncology will continue to follow the patient with yearly bloodwork and repeat imaging. Discussions regarding the next step in management are ongoing, with the understanding that if she develops symptoms or demonstrates enlargement of the metastatic lesions on imaging, the need for intervention becomes more acute.
DISCUSSION
The majority (~55–65%) of metastatic neuroendocrine tumors arise in the gastrointestinal tract [4], most of which are found in the small intestine; ~7.5% of gastrointestinal neuroendocrine tumor tumors are found in the colon [5]. The rate of metastatic neuroendocrine tumor at initial presentation is ~22% with half of those having unknown primaries [4]. Treatment for metastatic disease includes resection of primary tumors in patients that are appropriate candidates for surgery [5].
Management of neuroendocrine tumor metastases can be more challenging. Current treatment modalities include metastasectomy, cytoreductive surgery, hepatic arterial embolization, radiofrequency ablation and multiple medication-based regimens including somatostatin analogues and interferon alpha [5–7]. Radiofrequency ablation can be used in hepatic lesions with good outcomes, even near portal and hepatic veins, which act as a heat sink [8]. Irreversible electroporation (IRE) is another treatment option for metastatic disease to the liver and works by inducing cell death via disruption of the cell membrane with electrical pulses, sparing the extracellular matrix and surrounding tissue [9].
There are several portal vein branching variants and congenital anomalies described in the literature. Variants in normal branching pattern occur in as many as 20% of the population, with trifurcation of the main portal vein being the most common variant [10]. Agenesis of the main portal branches is the most common congenital anatomic variant of portal vein anatomy; however, this is frequently associated with lobar agenesis [10]. True absence of the portal vein bifurcation is a very rare anomaly, estimated to represent 0.3–2% of all portal vein anomalies [3].
There have been published series in which a hepatic resection has been performed for patients with an absent left portal vein with results varying from minor complications to death [12]. Although rare, neuroendocrine tumor crisis has been described during hepatic resection of metastatic neuroendocrine tumor, and portal vein occlusion is often performed during the operation to avoid vasoactive release from the tumor [13]. Due to this patient’s aberrant portal anatomy, metastasectomy becomes exceedingly difficult. For these reasons, the decision was made to perform an upfront resection of our patient’s primary tumor and subsequently pursue a multi-disciplinary team approach to her metastatic disease. If the lesions increase in size, the operative plan is lesion resection with preoperative radiofrequency ablation. The patient’s nearby portal vein should act as a heat sink during radiofrequency ablation and would be expected to remain patent. Despite the difficulty of the operation, studies have shown increased survival for patients undergoing metastasectomy for neuroendocrine tumors [11].
This case presents a challenging clinical scenario involving management of metastatic liver disease with aberrant portal vein anatomy. It is an extremely rare anatomic anomaly that has significant clinical consequences for the patient. The lesions abut the right portal vein, and resection risks compromise to global hepatic blood flow.
CONFLICT OF INTEREST STATEMENT
None declared.
References
Shuch, B, Bratslavsky, G, Linehan W.M., Srinivasan R. Sarcomatoid Renal Cell Carcinoma: A Comprehensive Review of the Biology and Current Treatment Strategies.
- magnetic resonance imaging
- computed tomography
- colonoscopy
- laparoscopy
- neoplasm metastasis
- neuroendocrine tumors
- patient care team
- surgical procedures, operative
- liver
- neoplasms
- midgut
- colectomy, right
- ascending colon
- right hepatic portal vein
- left hepatic portal vein
- interdisciplinary treatment approach



