-
PDF
- Split View
-
Views
-
Cite
Cite
Daniel P Bent, Robert S Boova, Minimally invasive mitral valve replacement after transcatheter edge-to-edge repair, Journal of Surgical Case Reports, Volume 2021, Issue 5, May 2021, rjab197, https://doi.org/10.1093/jscr/rjab197
Close - Share Icon Share
Abstract
Percutaneous transcatheter edge-to-edge mitral valve repair is available for treatment of both functional and degenerative mitral regurgitation (MR). This technique may be unsuccessful resulting in significant residual or recurrent MR. We described a successful minimally invasive mitral valve replacement in a patient with severe functional MR due to left ventricular dysfunction who previously underwent a transcatheter edge-to-edge repair.
INTRODUCTION
Transcatheter edge-to-edge repair (MitraClip, Abbot Laboratories, Abbott Park, IL) was approved by the US Food and Drug Administration for treatment of degenerative mitral regurgitation (MR) in prohibitive surgical risk patients in 2013 and approved for the treatment of functional MR in 2019 [1–3]. The approval for functional MR came after Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy (COAPT), a randomized controlled trial of patients with moderate–severe MR, showed a significant reduction in rate of hospitalization and mortality at 24 months [4]. Functional MR is diagnosed in the absence of structural or degenerative mitral valve (MV) disease and is usually due to left ventricular (LV) dysfunction with subsequent LV remodeling. Functional MR is associated with increased all-cause mortality and heart failure hospitalizations [5].
As utilization of percutaneous MV repair expands, procedural failure may require further intervention. We describe a case of recurrent severe MR in a high-risk patient with severe LV dysfunction who had previously undergone transcatheter edge-to-edge repair. We performed surgical clip extraction and MV replacement via a minimally invasive approach.
CASE REPORT
A 71-year-old female was referred to our institution for severe functional MR. The patient had a prior history of non-ischemic cardiomyopathy with implantable defibrillator, paroxysmal atrial fibrillation, chronic renal insufficiency, Crohn’s disease and chronic lung disease secondary to current tobacco use. The patient had multiple hospitalizations for congestive heart failure exacerbations and prior cardiac catheterization showing no obstructive coronary artery disease. At an outside institution, the patient underwent MitraClip implantation for her symptomatic MR. Two clips were placed with mean gradient 1.4 mmHg, mild residual MR, and left ventricular ejection fraction (LVEF) of 30% on a post-procedure echocardiogram. Four months post-procedure, the patient had a follow-up echocardiogram notable for two well-seated clips and mild MR.
Subsequently, the patient was brought to our institution with progressive shortness of breath and altered mental status. She was noted to be hypotensive requiring intravenous fluid, vasopressors and was treated for pneumonia. During that hospitalization, the patient had an echocardiogram showing LVEF 30% and moderate–severe MR with a mitral clip visualized (Fig. 1A). The patient was further evaluated with a dobutamine study that showed slight improvement in the LVEF and a hyperdynamic right ventricle. The patient recovered from this hospitalization, but her functional status continued to decline. She had an echocardiogram 3 months after her hospitalization, which showed worsening LVEF of 15–20% and severe MR likely secondary to worsening LV dilatation exacerbated by her acute illness (Fig. 1B). Given the ongoing symptoms and worsening LV function, our multidisciplinary team recommended MV replacement in an effort to improve symptoms, reduce the incidence of hospitalizations and preserve the remaining LV function.
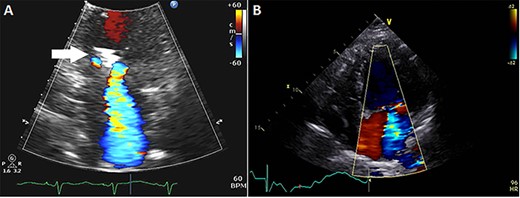
(A) Echocardiogram showing mitral clip (white arrow) at 4 months after clip placement; (B) at 7 months, left ventricular ejection fraction was 15–20%, diastolic and systolic diameters were 54 and 51 mm, respectively, and mean transmitral gradient 2.1 mmHg.
Eight months after MitraClip implantation, the patient underwent MV replacement via a minimally invasive right thoracotomy. The patient underwent cardiopulmonary bypass via the femoral vessels. Through a right thoracotomy, a left atriotomy was made. The previously placed mitral clips were associated with fibrotic changes and damage to the leaflets (Figs 2 and 3). The left ventricle was noted to be severely dilated. The MV was replaced with a 25-mm bovine pericardial bioprosthesis. Cardiopulmonary bypass was terminated at the conclusion of the operation with moderate inotropic support and satisfactory hemodynamic parameters. The intraoperative transesophageal echocardiogram showed mild improvement of LVEF from 20 to 30%. The bioprosthetic mitral valve was functioning appropriately without para-valvular leak and a transvalvular gradient of <2 mmHg. Postoperative transthoracic echocardiogram revealed an LVEF 30–35% and a normally functioning bioprosthetic MV with a mean transmitral gradient of 5 mmHg. The patient was extubated, weaned off inotropic support, and transferred out of the intensive care unit on post-operative days two, four and seven, respectively. She was ultimately discharged to home with home services. Her echocardiogram remained stable at time of discharge and 1 month follow-up. At 6 months postoperatively, the patient continued to progress with one brief hospital admission for non–cardiac-related concern.
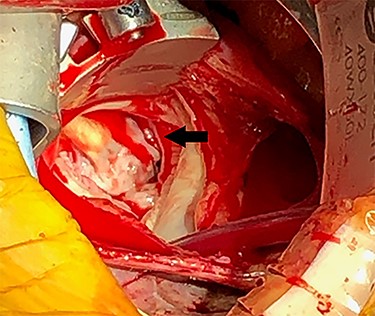
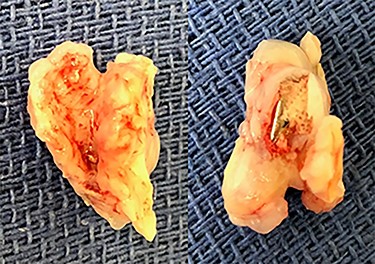
DISCUSSION
The Alfieri technique of edge-to-edge MV repair with suture approximation of the anterior and posterior mitral leaflets provided the framework for the development of the transcatheter approach using the MitraClip [6]. The Endovascular Valve Edge-to-edge Repair Study (EVEREST II) led to the approval of the MitraClip for degenerative MR [1]. In the EVEREST II trial, 23% of patients did not have acute procedural success prior to discharge and were referred to surgery [1]. At a 5-year follow-up, re-operation was significantly more frequent in the first year with percutaneous repair compared to surgical intervention [2]. With more experience, acute procedural success has improved as evidenced in the COAPT trial with a 96.6% rate of freedom from device-related complications at 12 months [4]. Nonetheless, there will still be patients requiring re-intervention.
MV repair with clip removal using a suture technique and median sternotomy has been previously described [7, 8]. Repeat percutaneous intervention may also be a viable option if MV leaflet coaptation is not compromised [9]. Subsequent operation has been associated with increased risk as there is a range of clip implantation-induced tissue damage [10]. Given the valvular changes associated with the clip, MV replacement may be more likely in comparison to MV repair [9–13]. A meta-analysis examining the optimal treatment strategy following failed surgical MV repair showed no significant differences between MV re-repair and replacement, although other studies have suggested less recurrence of MR with surgical replacement [14, 15]. We opted for surgical replacement of the valve due to two clips implanted compromising valve integrity and replacement was likely to be more durable.
Intervention after failed transcatheter edge-to-edge repair must be recognized early and requires multidisciplinary discussion as these are high-risk patients who were initially referred for a percutaneous approach. Minimally invasive MV surgery is a safe alternative to median sternotomy in high-risk patients as there is similar mortality rates and significantly lower rate of stroke [16]. In the event of unsuccessful percutaneous repair, MV surgery via a right thoracotomy can be considered as a less invasive and less traumatic approach in these high-risk patients.
ETHICS APPROVAL
The case report was reviewed by the Institutional Review Board at Temple University and was given a waiver. Informed consent was obtained.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
References
US Food and Drug Administration.



