-
PDF
- Split View
-
Views
-
Cite
Cite
LongHai Jin, Krishanth Naidu, Bouveret syndrome—a rare form of gastric outlet obstruction, Journal of Surgical Case Reports, Volume 2021, Issue 5, May 2021, rjab183, https://doi.org/10.1093/jscr/rjab183
Close - Share Icon Share
Abstract
Bouveret syndrome is a rare form of gastric outlet obstruction. It is typically diagnosed in frail elderly patients with protracted biliary disease. Thus, it has disproportionally high rates of morbidity and mortality. A 90-year-old man presented to our tertiary hospital with acute abdominal pain and symptoms of bowel obstruction. He was diagnosed with Bouveret syndrome on abdominal computed tomography and required judicious resuscitation and an emergency laparotomy. This article highlights the key features of Bouveret syndrome, and reviews the current diagnostic modalities as well as the contemporary treatment paradigm.
INTRODUCTION
Bouveret syndrome is an uncommon presentation of gastric outlet obstruction that has been reported only a limited number of times in the current literature. In contrast to its rarity, Bouveret syndrome has substantial morbidity and mortality rates due to complex patient and disease factors. Therefore, the choice of treatment must be carefully and timely selected with an appreciation of this complexity. This article presents a case of Bouveret syndrome in an elderly man at our tertiary hospital and discusses the intricacies associated with its treatment.
CASE REPORT
A 90-year-old man presented to our tertiary hospital with 3 days of severe upper abdominal pain, profuse vomiting and obstipation. This was on a background of recurrent post-prandial epigastric discomfort over the past decade. He has not had any infective or constitutional symptoms. He denied any history of obstructive jaundice. His past medical history was relatively insignificant with an open appendicectomy and an inguinal hernioplasty. On physical examination, there was no fever or haemodynamic compromise; however, he had a distended abdomen with epigastric tenderness.
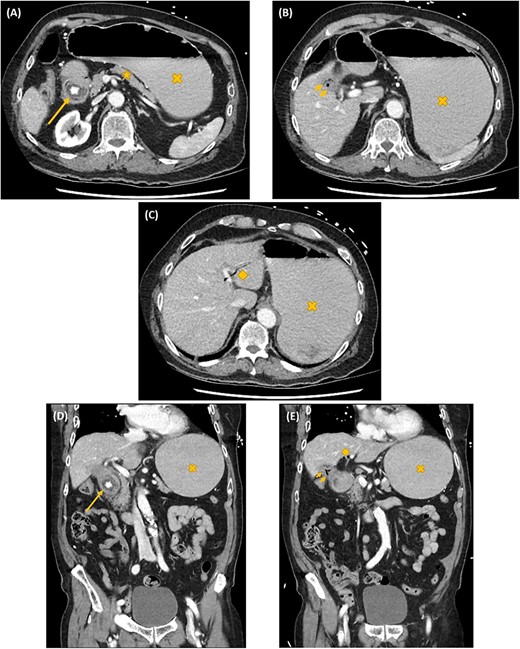
Axial (A–C) and coronal (D, E) computed tomography images showing gastric outlet obstruction (cross) due to a 3 cm hyperdense ectopic gallstone between the first and second parts of duodenum (arrow), with intrahepatic pneumobilia (diamond) and a decompressed gallbladder (arrow heads). This Rigler’s triad of findings was consistent with a cholecystoduodenal fistula and Bouveret syndrome.
On investigation, an acute inflammatory response, an acute kidney injury and a marked metabolic alkalosis were evident on biochemistry, but there was no cholestasis or hepatic dysfunction. Subsequent abdominal computed tomography (CT) identified a 3 cm hyperdense ectopic gallstone that was impacted between the first and second parts of the duodenum. There was gastric outlet obstruction proximal to this gallstone. A cholecystoduodenal fistula was noted with the presence of intrahepatic pneumobilia. These findings are collectively known as the Rigler’s triad (Fig. 1). Following initial resuscitative measures, an emergency laparotomy was undertaken. The operation was 3 h in duration and consisted of extensive adhesiolysis, duodenorrhaphy following stone extraction, subtotal cholecystectomy and finally an omental patch of the cholecystoduodenal fistula. On post-operative Day 6, his recovery was complicated with bilateral pulmonary emboli leading to both respiratory failure and obstructive shock. Despite thrombolysis and resuscitation, he succumbed 10 h later.
DISCUSSION
Gallstone ileus is an uncommon complication of cholelithiasis with an incidence of 0.3 to 0.5% [1–4]. Bouveret syndrome is even rarer as seen in only 1 to 3% of gallstone ileus [1–3]. This complication has been reported only 315 times over 50 years between 1967 and 2016 [5]. Bouveret syndrome specifically describes gastric outlet obstruction caused by an impacted gallstone at the pylorus or proximal duodenum [1–6]. The ectopic gallstone migrates via a bilioenteric fistula, which develops from chronic inflammation and pressure necrosis [5]. Cholecystoduodenal fistula is the most common form of bilioenteric fistula, as seen in about 60% of cases [4]. Choledochoduodenal and cholecystogastric fistula each comprise about 5% of cases [4]. Despite the rarity of Bouveret syndrome, its mortality rate is disproportionally high at 12 to 30% [3, 5, 7, 8]. This is related to its diagnosis typically in a geriatric population with protracted biliary disease, as well as frailty and comorbidities [5, 7]. The established risk factors for Bouveret syndrome are listed in Table 1 [9, 10].
The Rigler’s triad of gastric outlet obstruction, ectopic gallstone and pneumobilia on radiological imaging is pathognomonic for Bouveret syndrome [5, 7, 11]. This diagnosis can be established on CT, magnetic resonance cholangiopancreato-graphy (MRCP), ultrasound or upper gastrointestinal (UGI) endoscopy. CT is usually the most practical—its sensitivity for Rigler’s triad is between 75 and 78% [4, 11, 12]. One of the main limitations is its inability in visualizing isodense gallstones. One study reported that pneumobilia was evident in 60%; ectopic gallstone in 50%; and gastric outlet obstruction in 33% of cases with Bouveret syndrome on CT [13]. MRCP provides substantially improved diagnostic details on fistulous tracts, but its diagnostic potential is often limited by its accessibility in many clinical institutions. Ultrasound is useful for identifying gallstones and pneumobilia. Nevertheless, its sensitivity for Rigler’s triad is only 11%—intestinal distension and gas can make ultrasonography technically challenging [12]. It can also be tricky to localize the gallstone (orthotopic versus ectopic) with a collapsed gallbladder [12]. UGI endoscopy has the potential dual benefit of diagnosing and decompressing gastroduodenal distension simultaneously. However, the ectopic enteric gallstone and the enteric fistulous opening are visualized only in 69 and 31% of cases, respectively [5, 12, 13]. The success rate of endoscopic retrieval of the ectopic enteric gallstone is only about 10% [13].
Treatment of Bouveret syndrome can be broadly categorized into surgical and minimally invasive modalities. Surgical treatment involves gastric or enteric lithotomy via laparoscopy or laparotomy, with or without repair of cholecystoduodenal or choledochoduodenal fistula and cholecystectomy. Surgery has substantially higher success rates compared to the minimally invasive options—up to 90% for gastric or enteric lithotomy alone, and up to 82% if also including fistula repair and cholecystectomy [13]. Surgery can be performed as a single operation or as two operations with a staged procedure for fistula repair and cholecystectomy. The option of single operation has higher incidences of post-operative complications, but it has lower rates of recurrent retrograde biliary sepsis and gallstone ileus [1–3, 5]. The single- versus two-operation decision is made based on the degree of patient frailty, clinical illness severity and surgical complexity [1, 2, 5, 13].
Minimally invasive treatment includes endoscopic retrieval as well as laser, mechanical, electrohydraulic or extracorporeal shock wave lithotripsy. Although these options are associated with much lower complication rates, but their success rates are typically around 10 to 25% [13]. Lithotripsy can cause migration of fragments along the gastrointestinal tract and result in distal obstruction [1]. Nevertheless, these options provide useful alternatives when patients cannot have surgery [5, 13]. As technology continues to advance, the success rates of these modalities will also continue to improve in the future.
| Risk factors for Bouveret syndrome . |
|---|
| Old age (>60 years) |
| Female gender |
| Large gallstones >2 cm |
| Recurrent biliary colic and chronic cholecystitis |
| Post-surgical altered gastrointestinal anatomy |
| Risk factors for Bouveret syndrome . |
|---|
| Old age (>60 years) |
| Female gender |
| Large gallstones >2 cm |
| Recurrent biliary colic and chronic cholecystitis |
| Post-surgical altered gastrointestinal anatomy |
| Risk factors for Bouveret syndrome . |
|---|
| Old age (>60 years) |
| Female gender |
| Large gallstones >2 cm |
| Recurrent biliary colic and chronic cholecystitis |
| Post-surgical altered gastrointestinal anatomy |
| Risk factors for Bouveret syndrome . |
|---|
| Old age (>60 years) |
| Female gender |
| Large gallstones >2 cm |
| Recurrent biliary colic and chronic cholecystitis |
| Post-surgical altered gastrointestinal anatomy |
The patient in our case was offered an operation rather than endoscopic alternatives in view of his abdominal peritonism, significant inflammatory response and end-organ dysfunctions, minimal medical comorbidities, perceived superior treatment outcome with surgery, availability of an upper gastrointestinal-hepatobiliary surgeon as well as support from the anaesthetist and intensivist. As the physiology of our patient remained reasonably robust throughout the operation, a single-stage operation was thus performed with fistula repair and cholecystectomy.
CONFLICT OF INTEREST STATEMENT
None declared.



