-
PDF
- Split View
-
Views
-
Cite
Cite
Anya L Greenberg, Won-Tak Choi, Oren Shaked, Anthony T Lee, Iman K Berrahou, Line G Jacques, Carter C Lebares, Appendiceal neurofibroma in a patient with neurofibromatosis 1 and recurrent abdominal infections from ventriculoperitoneal shunt: a case report, Journal of Surgical Case Reports, Volume 2021, Issue 4, April 2021, rjab115, https://doi.org/10.1093/jscr/rjab115
Close - Share Icon Share
Abstract
Appendiceal neurofibromas are exceedingly rare, with neither experimental nor observational data to support evidence-based diagnosis or treatment. We describe the case of a 52-year-old woman with neurofibromatosis 1 (NF1) complicated by aqueductal stenosis and resultant hydrocephalus needing a ventriculoperitoneal shunt (VPS). She presented to the emergency department with abdominal pain and was found to have abnormalities in the right hemiabdomen on cross-section imaging, also a Staphylococcus epidermidis growth at the distal portion of the VPS. She was initially treated with two rounds of intravenous antibiotics and VPS removal without improvement. She ultimately underwent an appendectomy, which revealed pathologic evidence of NF. The appendectomy was key to ruling out malignancy, addressing further symptoms and preventing future malignant transformation. This case highlights the importance of including appendiceal neurofibromas in the differential diagnoses of abdominal pain in patients with NF1.
INTRODUCTION
Neurofibromatosis (NF) is a group of genetic disorders characterized by tumors of the nervous system, or neurofibromas [1]. The most common type is NF1, an autosomal dominant disorder that occurs in 1 in 2500–3000 births [2]. Manifestations include ‘café-au-lait’ spots, cutaneous neurofibromas, Lisch nodules and axillary and inguinal freckling [3].
Mutations in the neurofibromin 1 (NF1) gene result in deficiency of neurofibromin, a protein involved in tumor suppression [4, 5]. The risk of malignancy in NF1 patients has been shown to be nearly three times higher than that of the general population [6]. Moreover, while most neurofibromas themselves are benign, 8–12% undergo malignant transformation [7].
Up to 25% of patients with NF1 have neurofibromas in the gastrointestinal (GI) tract. Most frequently affected are the small intestine, retroperitoneum and colon [8]. Appendiceal neurofibromas are exceedingly rare, with only 11 cases reported in the literature to our knowledge [7, 9–11]. Most of these patients presented with abdominal pain and were thought to have appendicitis. Some initially were treated medically, but all ultimately had surgical resection [7, 9–11]. Here, we present the case of a patient with appendiceal neurofibroma.
CASE REPORT
A 52-year-old woman presented to the emergency department (ED) with altered mental status. She had a history of NF1 complicated by aqueductal stenosis and resultant hydrocephalus for which a ventriculoperitoneal shunt (VPS) was placed 13 years prior. She subsequently underwent multiple VPS revisions for recurrent infections, most recently 3 weeks prior to this admission.
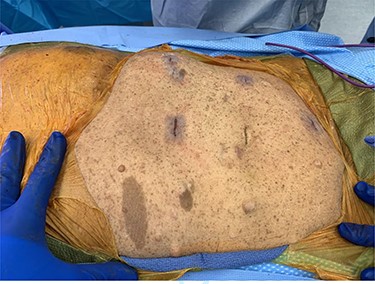
At that time, she presented to the ED with 2 weeks of abdominal pain. She was afebrile and hemodynamically stable. On examination, she had tenderness in the right upper quadrant (RUQ) and was noted to have diffuse cutaneous neurofibromas (Fig. 1). Her labs were unremarkable, except elevated alkaline phosphatase (137 U/l) and alanine transaminase (146 U/l). Computerized tomography of the abdomen and pelvis (CTAP) revealed a perihepatic cyst around the shunt catheter tip, appendiceal and peritoneal thickening, stranding along the right hemiabdomen and diffuse peritoneal nodularity with partial calcification (Fig. 2). Upon laparoscopic exploration and cyst unroofing, the patient was noted to have intra-abdominal inflammatory adhesions in the right hemiabdomen. Cerebrospinal fluid (CSF) cultures from the distal portion of shunt grew Staphylococcus epidermidis, and the patient was treated with intravenous antibiotics.
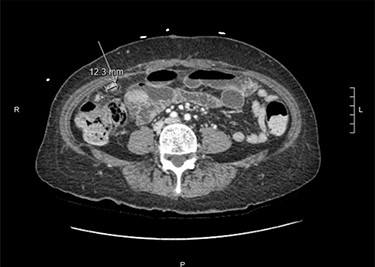
Computerized tomography of the abdomen and pelvis demonstrating appendiceal and peritoneal thickening.
Two weeks later, she became confused and re-presented to the ED. Per her family, she had decreased appetite and poor oral intake since discharge. She had been complaining of abdominal pain for several days and had one episode of emesis. At presentation, she was afebrile, hypertensive (140/92) and tachycardic (125 bpm). On examination, her abdomen was tender in the RUQ, right lower quadrant (RLQ) and suprapubic region, and she was oriented to place only. Laboratory studies were notable for white blood cell count of 12.1/mm3 with neutrophil predominance, mildly elevated alkaline phosphatase (149 U/l) and international normalized ratio (1.6), and persistent CSF with S. epidermidis. CTAP revealed a fluid collection in the RLQ with a tract connecting to the tip of the VPS catheter; scattered calcified nodules within the omentum, peritoneum and along the sigmoid colon; and appendiceal thickening. The patient was started on another course of intravenous antibiotics. The VPS was explanted shortly after admission and an external ventricular drain was placed.
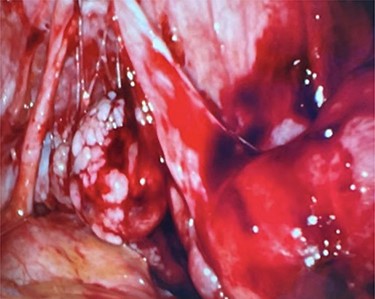
Right ovary with papillary excrescences and grossly abnormal appendix.
Due to concern about the fluid collection in the RLQ, a CTAP was repeated 5 days after admission and again 5 days later, revealing interval resolution of the collection but otherwise no change in the abnormal RLQ anatomy, raising suspicion for appendiceal versus gynecologic neoplasm. As such, the patient underwent a diagnostic laparoscopy and nodular biopsy.
On entering the abdomen, the terminal ileum and cecum were grossly normal, while the right ovary showed papillary excrescences on the serosal surface (Fig. 3). The appendix was proximally distended with distal serosal injection and chronic scarring into the pelvis (Fig. 3). An appendectomy was performed (Fig. 5A). Scattered peritoneal nodules were visualized on the abdominal wall anterior to the uterus (Fig. 4), and several biopsies were taken. Cut sections of the appendix revealed uniformly firm, white tissue throughout the entire specimen with a possible centrally located, pinpoint lumen, suggesting fibrous obliteration. No well-circumscribed lesion was identified; however, histopathology showed the appendiceal wall was diffusely expanded by an irregular, wavy spindle cell proliferation involving the mucosa, submucosa, muscularis propria and subserosal tissue (Fig. 5B and C). Immunohistochemical stains highlighted a mixture of S100/SOX10-positive cells and CD34-positive cells, consistent with appendiceal neurofibroma (Fig. 5D). Pathologic diagnosis of the peritoneal biopsies was low-grade serous neoplasia.
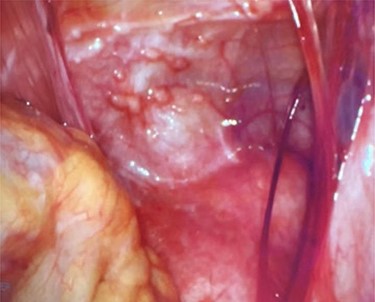
Abdominal wall anterior to the uterus with scattered peritoneal nodules.
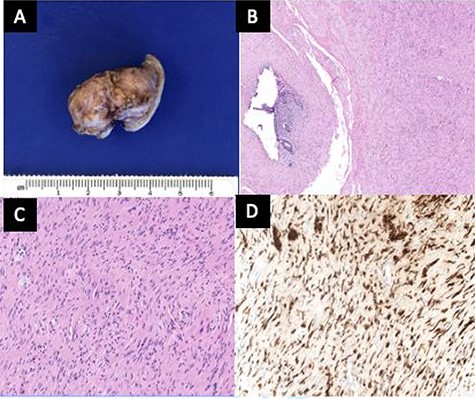
(A) The outer surface of the appendix was smooth to slightly rough with cauterized fibrous adhesions. (B) The appendiceal wall was diffusely expanded by an irregular spindle cell proliferation involving the mucosa, submucosa and muscularis propria. A tiny lumen was identified in this section, but the other sections showed completely obliterated central lumen, mimicking fibrous obliteration. (C) High-power view of spindle cells in the muscularis propria demonstrated wavy dark nuclei. (D) Spindle cells were positive for S100.
DISCUSSION
Due to the low incidence of appendiceal neurofibroma, neither experimental nor observational studies have been conducted to guide an evidence-based approach to diagnosis or treatment. However, the standard of care in symptomatic neurofibroma in the GI tract involves surgical resection due to the high incidence of malignancy and possibility of malignant transformation [12]. This line of reasoning has been applied to previously documented cases of appendiceal neurofibroma, whereby despite not having the correct preoperative diagnosis, it was determined retrospectively that surgical excision was the appropriate course.
For our patient, non-specific signs and symptoms of appendiceal neurofibroma (e.g. abdominal pain, appendiceal thickening) were present at her first ED presentation. However, given the bacterial growth in the perihepatic fluid, the intra-abdominal infectious process was thought to be the cause of her symptoms and the patient was treated medically. Consistent with the other reported cases [7, 9–11], continued clinical vigilance and resultant exploratory laparoscopy with appendectomy were critical in ruling out malignancy, addressing further symptoms and preventing future malignant transformation. This case highlights the importance of including appendiceal neurofibromas in the differential diagnoses of abdominal pain in patients with NF1, even if another plausible explanation is present.
Notably, while the association between NF1 and ovarian cancer has not been established, emerging evidence suggests that a link may exist [4]. In addition to appendiceal neurofibroma, our patient was found to have low-grade serous carcinoma. Further investigation is needed to determine whether this gynecologic finding may be related to NF1, thus potentially warranting additional screening in this population, or if it is an independent process given the overall low incidence of ovarian cancer in patients with NF1 [13].
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
References
- abdominal pain
- hydrocephalus
- appendectomy
- cancer
- emergency service, hospital
- neurofibroma
- neurofibromatosis 1
- staphylococcus epidermidis
- diagnosis
- diagnostic imaging
- ventriculoperitoneal shunt
- abdominal infections
- evidence-based practice
- antibiotic therapy, intravenous
- malignant transformation
- participation in ward rounds
- aqueductal stenosis



