-
PDF
- Split View
-
Views
-
Cite
Cite
Michael J Minarich, Leonard R Henry, Ashley Hardy, Urs von Holzen, Management of post-operative subcutaneous emphysema with the application of wound VAC therapy, Journal of Surgical Case Reports, Volume 2021, Issue 4, April 2021, rjab103, https://doi.org/10.1093/jscr/rjab103
Close - Share Icon Share
Abstract
Extensive subcutaneous emphysema (SE) complicates between 1 and 6% of elective thoracic procedures. The management of SE is varied, and may include increasing the suction of chest tubes, placement of additional chest tubes, placement of subcutaneous drains and creation of releasing incisions. We present five patients with post-operative SE treated successfully with a subcutaneous infraclavicular incision and wound VAC therapy. A 5-cm incision was made 2 cm below the clavicle down and through the pectoralis major fascia. A VAC dressing was fitted to the wound and suction was applied to −125 mm Hg. Data were retrospectively collected and analyzed. VAC dressing was placed a median of 6 days after initial operation. All patients had improvement in symptoms and resolution of SE by VAC dressing therapy. Subcutaneous infraclavicular incision and VAC dressing placement is a viable treatment for patients with post-operative SE who fail conservative therapy.
INTRODUCTION
Subcutaneous emphysema (SE) complicates between 1 and 6% of elective thoracic procedures [1, 2]. SE is frequently found in the absence of a concomitant pneumothorax, and is felt to be a direct draining of a post-operative air leak into the previous VATS or thoracotomy wound, or the mediastinal structures rather than the pleural space, where the chest tube can drain the air. As the air leak dissects through the subcutaneous, subfascial or mediastinal tissue planes, it may cause swelling on the chest, arms, neck and up to the periorbital region, temporarily affecting both voice quality and sight in severe circumstances. The typical onset of post-operative SE is at 3 days. [3].
The management of SE is varied and may include increasing the suction of in situ chest tubes [2], repositioning of in situ chest tubes, placement of a second or additional tubes for improved drainage [2], placement of subcutaneous drains [4] and creation of infraclavicular incisions to release subcutaneous air [5]. Increasing the suction on the previously placed chest tube to −40 cm H2O has been reported to resolve SE in two thirds of patients [2]. More invasive treatment is used in the third of patients, where increasing the suction fails. Although previous studies have shown success in resolving SE with subclavicular blowhole incisions, we combined this approach with placement of a wound VAC dressing to decrease the time to resolution of symptoms.
CASE SERIES
A total of five patients were identified in the post-operative setting as having SE that worsened despite increasing the suction on the operatively placed chest tube. All patients were highly symptomatic with shortness of breath, increasing oxygen requirements and voice changes.
A 5-cm incision was made 2 cm below the clavicle on the ipsilateral side of the thoracic procedure under local anesthesia at the bedside. The incision was deepened through both the subcutaneous tissue and the pectoralis major fascia. Frequently, bubbling was encountered in the wound as the subcutaneous tissues started immediately decompressing. A black wound VAC sponge was cut to size and placed within the wound (Fig. 1A and B). The included plastic sheeting was used to form an adequate seal and suction was applied to −125 mm Hg (V.A.C.ULTA™ system while in-patient, then switched to the ACTIV.A.C.™ system at discharge, KCL/3M, 3M Center, St Paul, MN 55144-1000, USA). Once the wound VAC was placed it was very well tolerated, and only minimal pain medication was required. Sponge and dressing changes were performed twice a week until removal. After the SE had resolved, the wound VAC was removed in the clinic. Upon removal, nice granulation tissue was present and the wound was loosely adapted by interrupted sutures. All wounds healed without complications and the sutures were removed 7–10 days later.
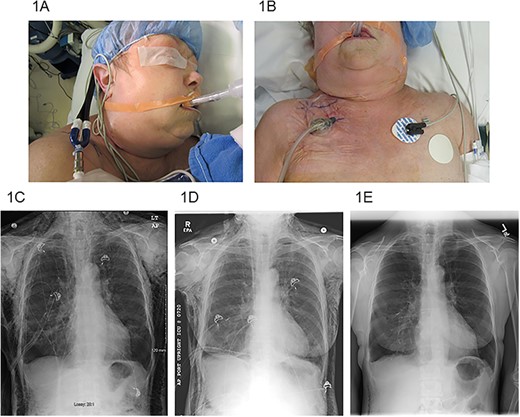
(A) SE extending to the periorbital region, (B) right infraclavicular incision location with VAC dressing, (C) pre-operative chest X-ray, (D) chest X-ray post-operative day #3 after VAC placement and (E) chest X-ray post-operative day #22 after VAC placement.
Three of the patients were male and two were female. The median age was 68 years (range 61–70). All patients had an extensive smoking history. All patients were treated with VATS resections: one wedge resection, one lobectomy, two combined lobectomy and wedge resections for NSLC, and one thymectomy with extensive adhesiolysis for a thymoma. Chest tubes were routinely placed on −20 cm H2O suction post-operatively. Initial efforts at management of SE included both increase in chest tube suction (range 25–40 cm H2O) and slight withdrawal of the chest tube (range 2–5 cm). The VAC dressing was placed a median of 6 days after the initial operation (range 3–19 days). Median overall length of stay was 16 days (range 7–23). Median length of VAC treatment was 10 days (range 4–15). All patients had immediate improvement in symptoms and quick resolution of SE by the VAC dressing therapy (Fig. 1C–E). All patients were successfully discharged home. One patient was discharged without a chest tube or VAC therapy, one patient required a chest tube with Heimlich valve, two patients required only VAC therapy, and one patient required both VAC therapy and chest tube with Heimlich valve at discharge. No complications of incision or VAC dressing placement were observed.
DISCUSSION
Management of post-operative SE is an uncommon problem for patients undergoing elective thoracic procedures. Although generally not life threatening, the swelling of the neck and face due to SE can affect the patient’s voice and vision, causing discomfort, compromise the airway, and potentially eroding trust in the surgical care being provided.
The majority of patients have successful resolution of their post-operative SE with increase in suction on their operatively placed chest tube. For those whose SE does not improve, more invasive treatment is warranted. The placement of a second chest tube is not always efficacious, as only a quarter of patients with recalcitrant SE have an air leak in their existing chest tube [2]. This might be due to the seal the air leak forms directly to the incision site rather than the pleural space, or direct mediastinal tracking, and can be better addressed with either an operative procedure to disrupt the fistula tract, or by a bedside procedure to release the SE and resolve the symptoms until the air leak itself heals. Herlan et al. described creating 3-cm bilateral infraclavicular incisions down to the level of the pectoralis fascia as a way to relieve the symptoms of SE [5]. Recognizing that infraclavicular incisions functioned only until the wounds would seal with blood clots, Beck et al. proposed inserting perforated catheters into the subcutaneous space to drain the air and relieve the symptoms [4]. Although these catheters were also limited by clotting, their ease in placement and minimal disfigurement were felt to be a benefit over the blowhole approach.
Recognizing the limitation of both methods, we utilized infraclavicular incisions combined with a wound VAC dressing at −125 mm Hg suction to quickly resolve the SE, and keep the wound patent with evacuation of any bleeding or fluid with the VAC dressing. This successfully treated all five cases of recalcitrant SE in our patient cohort. This procedure can safely be performed at the bedside under local anesthetic with minimal patient discomfort. As VAC therapy can be used to treat infected or contaminated wounds, the risk of wound infection after this procedure is minimal, and we found no evidence of wound infection in any of the patients treated. Furthermore, placement of wound VAC therapy may be able to obviate the need for continued chest tube management for some patients with post-operative SE.
In conclusion, subcutaneous infraclavicular incision and wound VAC dressing placement is a viable treatment option for patients with post-operative SE who fail conservative therapy. The procedure is easy to perform at the bedside under local anesthesia, and the wound VAC dressing is easy to manage with minimal risk of clotting or wound infection. In some patients, this may even allow for management of post-operative pulmonary air leak without the need for concurrent chest tubes.
DECLARATIONS
There was no outside support for this work. A waiver for this study was obtained by the local IRB.
CONFLICT OF INTEREST STATEMENT
There are no personal conflicts of interest or financial conflicts of interest regarding this manuscript by any of the authors.



