-
PDF
- Split View
-
Views
-
Cite
Cite
Kevin Xiao, Sharon Swierczynski, Gary Xiao, Small, low-grade ampullary neuroendocrine tumor presenting with metastasis and multiple synchronous tumors in a patient with neurofibromatosis type 1: a case report with literature review, Journal of Surgical Case Reports, Volume 2021, Issue 3, March 2021, rjab076, https://doi.org/10.1093/jscr/rjab076
Close - Share Icon Share
ABSTRACT
Neurofibromatosis type 1 (NF1) is a tumor syndrome and one of the most common genetic disorders. Patients have an increased risk of developing neurologic and gastrointestinal (GI) neoplasms, but GI lesions are often underrecognized since most cases are asymptomatic. It is extremely rare to see multiple types of abdominal tumors synchronously in NF1. In this case, we describe a patient presenting with a small, low-grade periampullary neuroendocrine tumor (NET) that underwent endoscopic submucosal dissection and later pancreaticoduodenectomy (Whipple procedure). This led to findings of lymph node and distant metastasis of her NET, and the incidental discovery of gastrointestinal stromal tumors, extensive pancreatic intraepithelial neoplasia, and main duct and side branch intraductal pancreatic mucinous neoplasm. The synchronous presence of these lesions has not been reported in the literature.
INTRODUCTION
Patients with neurofibromatosis type 1 (NF1) are prone to developing a variety of gastrointestinal (GI) tumors that often go unrecognized. Presentation of symptoms varies greatly depending on the location and the type of the lesion. Neuroendocrine tumors (NETs) in patients with NF1 most commonly affect the duodenum. Periampullary NETs often present with symptoms of biliary obstruction, and management typically includes resection. During workup and management, additional considerations should be given to the possibility of metastasis and the synchronous presence of other types of GI neoplasms associated with NF1.
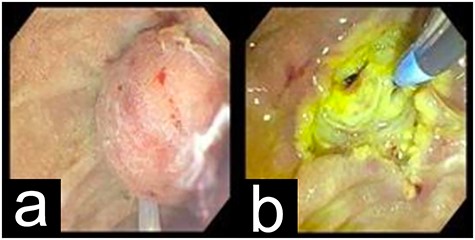
EGD showing the periampullary mass before (a) and after (b) resection.
CASE PRESENTATION
A 50-year-old woman presented with abnormal liver function tests after routine bloodwork. Her history was notable for NF1, type 2 diabetes mellitus and dyslipidemia. She had a previous surgical history of laparoscopic cholecystectomy, appendectomy, hysterectomy and left salpingo-oophorectomy. Labs showed an AST of 68 IU/l, ALT of 95 IU/l, alkaline phosphatase of 394 IU/l and total bilirubin of 1.1 mg/dl.
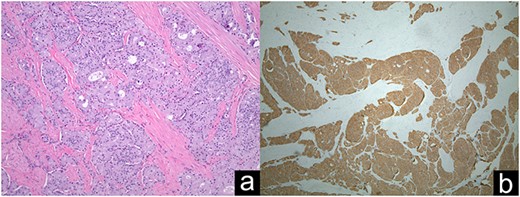
Ampullary well-differentiated, low-grade (G1) neuroendocrine tumor (a); Synaptophysin staining in immunohistochemistry (b).
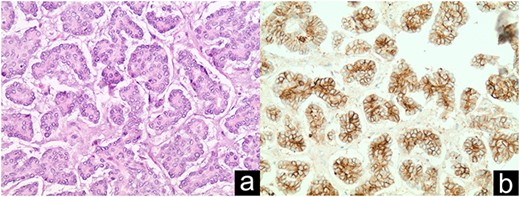
Distal NET metastasis to umbilical hernia sac discovered intraoperatively (a); CD56 staining in immunohistochemistry (b).
Initial ultrasound revealed significant intrahepatic and extrahepatic biliary dilation. Fatty liver was first considered due to her obesity. Despite over 40 pounds of weight loss over the next 2 months, labs continued to rise, and she had developed pruritis. She had an AST of 343 IU/l, ALT of 295 IU/l, alkaline phosphatase of 590 IU/l and total bilirubin of 1.6 mg/dl. Abdominal CT revealed worsening dilation of the main pancreatic duct to 6 mm and common bile duct dilation to 25 mm suggesting an ampullary soft tissue mass protruding into the duodenum.
The patient underwent esophagogastroduodenoscopy (EGD) and endoscopic ultrasound with fine-needle aspiration (FNA), revealing a 21 × 17 mm polypoid ampullary mass causing biliary and pancreatic duct obstruction (Fig. 1). FNA biopsy initially came back as polypoid duodenitis. The patient was referred for endoscopic submucosal dissection where a 20-mm submucosal mass was found in the papilla major and removed. Pathology came back for well-differentiated, low-grade G1 (based on 2010 WHO Criteria) 1.1-cm ampullary neuroendocrine tumor staining with a positive posterior margin (Fig. 2).
At this point, the patient was presented with two options: lifelong surveillance with EGD or Whipple procedure. Ultimately, the patient elected for Whipple. An umbilical hernia was discovered during her preoperative office visit that she opted to have repaired simultaneously.
Intraoperatively, two small nodules were found and removed from the surface of the liver. The frozen section came back with focal nodule hyperplasia. During resection of the proximal jejunum, a 10-mm polypoid mass was appreciated on the surface. The umbilical hernia sac was also removed.
Pathology report revealed the distant umbilical sac was positive for a 2.8-cm metastatic NET (Fig. 3). Four of 19 peripancreatic lymph nodes were positive for metastatic NETs (Fig. 4). Incidental gastrointestinal stromal tumor (GIST) turmorlets less than 1 cm in size were identified in the proximal jejunum (Fig. 5). There was extensive PIN Grade 1–2 found at the pancreatic neck margin and main duct intraductal pancreatic mucinous neoplasm (IPMN) with dysplasia and side branch IPMN (Fig. 6).
The patient’s postoperative recovery course was uneventful. Positron emission tomography/computed tomography (PET/CT) was planned, followed by routine imaging surveillance to monitor for additional metastasis.
DISCUSSION
The incidence of GI tumor involvement in NF1 has been reported in 10–25% of patients. However, only 5% of patients report GI symptoms. Manifestations of these lesions are highly variable depending on the location and extent of tumor involvement [1, 2].
Periampullary duodenal NETs (dNETs) are extremely rare but are associated strongly with NF1. Although they often stain for somatostatin reactivity, they are typically nonfunctional and rarely demonstrate symptoms of somatostatinoma syndrome. Instead, they display signs of biliary and pancreatic duct obstruction [1–3].
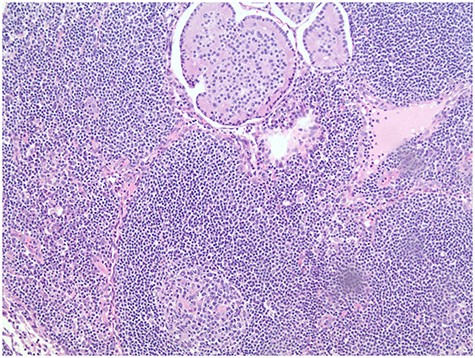
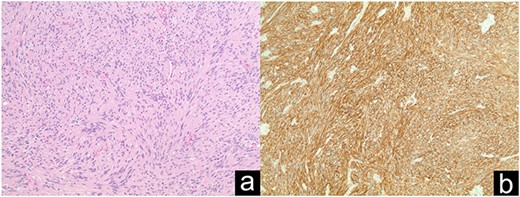
GIST discovered incidentally intraoperatively (a); DOG1 staining in immunohistochemistry (b).
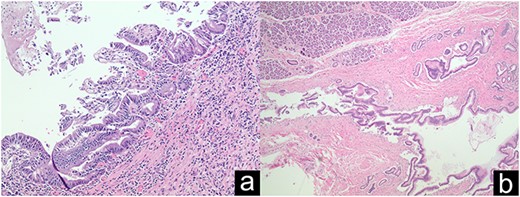
Grade 1–2 PIN at the pancreatic neck margin (a); Low power view of main duct IPMN (b).
The behavior of ampullary versus non-ampullary dNETs is under debate. Older studies report that ampullary dNETs show no association between size and metastases, while non-ampullary dNETs > 2 cm demonstrate increased risk for locoregional and distant metastases at initial presentation. More recent studies report that dNETs, regardless of whether they are ampullary or non-ampullary, demonstrate odds of lymph node (LN) metastasis are increased with increasing tumor size > 1 cm but not associated with tumor grade [2–4].
Management of dNETs typically includes ESD, local resection, or Whipple, depending on tumor characteristics. Both anatomical and local resections are associated with increased survival compared to no intervention. Increasing tumor size > 2 cm and high grade were associated with decreased survival. Recurrence after resection was reported to be increased with increasing tumor size > 1 cm and high grade. Interestingly, there were no reported differences in recurrence between endoscopic mucosal, local resection or Whipple procedure [2, 5, 6].
GIST is associated with a 200-fold increase in prevalence in NF1 patients compared to the general population. It is the most common GI specific tumor in NF1. Treatment in NF1 typically includes resection since its molecular characteristics make it resistant to tyrosine kinase inhibitors [1, 7].
IPMN and PIN are known precursor lesions to pancreatic ductal adenocarcinoma (PDAC). PIN is the most common precursor lesion and the risk of progression to invasive PDAC increases with grade. Main duct IPMNs have the highest malignant potential and risk of progression to invasive PDAC [8, 9].
Despite their small size, low-grade dNETs can still present with metastasis at initial presentation. Our patient presented with a 1.1-cm primary lesion but demonstrated both LN and distant metastasis. Tumor size is important to consider due to increased rates of LN metastasis and recurrence and decreased survival with increasing size. However, the rates of distant metastasis are poorly characterized for dNETs in the current literature. We found several other unexpected lesions intraoperatively, including GIST, main duct and side branch IPMN and PIN grade 1–2. Per our literature review, there was no case reported like ours demonstrating small, low-grade dNETs presenting with LN and distant metastases as well as several incidental findings of synchronous GI tumors and precancerous lesions.
In conclusion, the workup of small and low-grade NETs should be comprehensive. It is also important to consider the possibility of metastasis at initial presentation and the possibility of other synchronous tumors and lesions in these patients during preoperative work up, resection and postoperative pathological examination.



