-
PDF
- Split View
-
Views
-
Cite
Cite
Dalibor Sila, Markus Lenski, Stefan Rath, Giant internal carotid aneurysm: endovascular parent artery occlusion after failed treatment using a flow diverter—case report, Journal of Surgical Case Reports, Volume 2021, Issue 2, February 2021, rjab015, https://doi.org/10.1093/jscr/rjab015
Close - Share Icon Share
Abstract
Treatment of giant aneurysms is challenging. While parent vessel reconstruction is the primary therapeutical target, the parent artery occlusion (PAO) is considered the next treatment option. We report a case of a 56-year-old woman with a right-sided non-ruptured giant aneurysm of the cavernous internal carotid artery. After failed aneurysm treatment by vessel remodeling through a flow diverter stent, we decided upon aneurysm coiling and PAO. Prior to the procedure, a successful balloon occlusion test (BOT) was performed, and in the second stage, just before occluding the parent artery, the BOT with induced hypotension was repeated. We achieved a good angiographic result and successful outcome without neurological deficit. In the case of failed treatment of giant aneurysm by vessel reconstruction, PAO is a therapeutical option. Prior to the vessel occlusion, a BOT with induced arterial hypotension challenge should be performed.
INTRODUCTION
The outcome of patients with giant aneurysms is rather poor due to the high risk of bleeding, neurological deficits caused by mass effect and thromboembolic complications yielding to cerebral ischemia. According to ISUA (Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment) Trial, the annual risk of bleeding from a giant aneurysm is 8% with a cumulative 5-year risk of rupture is 40–50% depending on the localization. Aneurysm size together with localization in posterior circulation is the predictor of poor outcomes in case of rupture [1]. Treatment of giant aneurysms is challenging. While vessel reconstruction is the primary therapeutical target, the parent artery occlusion (PAO) is considered the next treatment option.
CASE REPORT
A 56-year-old woman without significant prior medical history presented with ptosis on the right side as a sign of external oculomotor palsy. Magnetic resonance (MR) imaging investigation confirmed a large mass lesion of 30 mm in diameter parasellar on the right side without perifocal edema. In MR angiography, we suspected a giant aneurysm of cavernous internal carotid artery (ICA). In the digital subtraction angiography (DSA), a giant cavernous part ICA aneurysm with wide contact to parent vessel was confirmed (Fig. 1a–d). We decided on treatment by vessel reconstruction using Pipeline™ flow diverter stent. This treatment failed after incorrect unfolding of the sheet stent in the curved part of the vessel just proximal to aneurysm neck. Several attempts to unfold the stent failed. For this reason, the intervention had to be terminated without success.
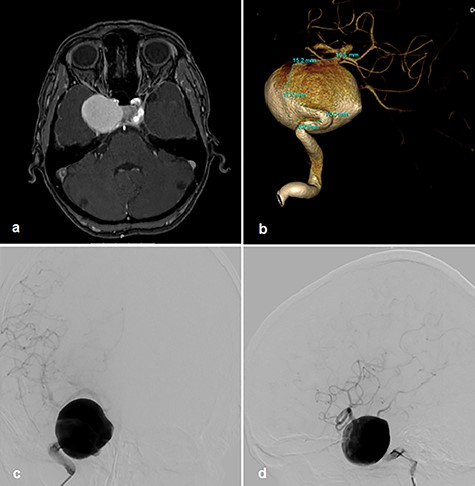
Right cavernous ICA Aneurysm on MR TOF angiography (a); DSA 3D angiogram (b); p–a projection angiogram (c); lateral angiogram (d).
Therefore, the decision was made to use parent vessel occlusion. Initially, we performed a balloon occlusion test (BOT) with clinical evaluation as well as cerebral blood flow measurement by transcranial Doppler sonography (TCD). Initially, the systolic flow on the TCD in proximal medial cerebral artery (MCA) was 110–120 cm/s. Occlusion of the right ICA proximal to the stent with balloon a period of 30 min was performed. Left ICA and vertebral artery angiograms showed collateral perfusion of right hemisphere (Fig. 2a and b). There were no clinical symptoms over the time of BOT, but systolic flow reduction in the proximal MCA was observed up to 80 cm/s in the TCD.
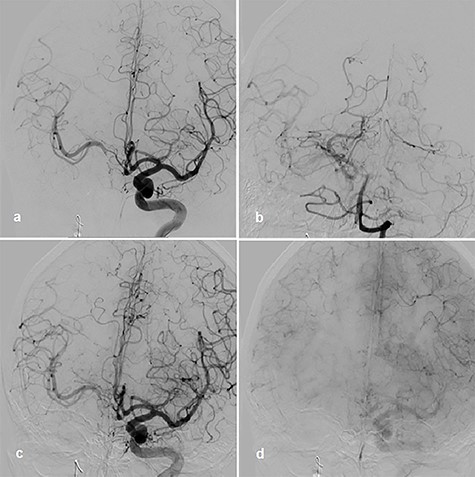
BOT, left ICA angiogram with right-sided collateral perfusion (a); collateral perfusion via posterior communicating artery (b); left ICA angiogram during BOT and hypotensive challenge, arterial phase (c); venous phase (d).
Afterward, we decided to perform aneurysm coiling and PAO. Prior to this, we repeated the occlusion test with clinical, angiographic and TCD control before and during induced arterial hypotension. In stand-by anesthesia with central venous catheter and invasive blood pressure monitoring, a 4-F catheter was placed in the left ICA and a 6-F guiding catheter was placed in the right ICA. Occlusion of the right ICA was performed with a balloon. With 100 mmHg mean blood pressure, we measured 78 m/s systolic flow in the TCD on the right MCA. The patient presented no neurological symptoms. Left ICA angiogram showed good crossflow perfusion of the right side over the anterior communicating artery. After a drop of mean blood pressure from 100 to 70 mmHg using intravenous administration of urapidil, there was no neurological deficit observed over the period of 30 min. Furthermore, there was no perfusion reduction and no delay of venous phase on the right side on the angiogram of left ICA (Fig. 2c and d), but we observed systolic flow reduction from 78 to 50 cm/s on the right MCA in the TCD during induced arterial hypotension. Based on the results of good collateral flow during occlusion test without neurological deterioration after hypotension challenge, we finally performed PAO by coiling with seven coils (Fig. 3a).
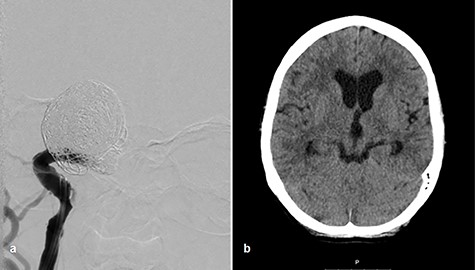
Coiling of giant aneurysm and parent vessel occlusion, p–a angiogram (a), CT scan on Day 1 (b).
After aneurysm coiling with PAO, the patient was put on dual antiaggregation therapy with aspirin 100 mg and clopidogrel 75 mg per day for the next 6 weeks, followed by aspirin alone 100 mg per day lifelong. Computed tomography (CT) scan on Day 1 presented no infarction (Fig. 3b). Perfusion CT scan on Day 9 after intervention showed regular perfusion of the right hemisphere without any cerebral blood flow/cerebral blood volume mismatch. The oculomotor palsy had subsided after 3 months.
DISCUSSION
Treatment options of unruptured giant aneurysms are endovascular, microsurgical, combined or conservative. Endovascular treatment strategy can be destructive (parent vessel occlusion), but the development of materials like stents and coils allows vessel reconstruction to be a primary endovascular target [2, 3]. Becske et al. [2] (PUFS trial) reports the complete aneurysm obliteration after vascular remodeling using flow diverter device pipeline in 93.4% of cases without recanalization in a 3-year follow-up. Long-term neurological morbidity and mortality was 5.6%. In the ASPIRe trial, the overall morbidity and mortality totals 6.8%. Complete occlusion rates were 76% at the last follow-up [4].
Another endovascular treatment of giant aneurysms includes parent vessel occlusion. Labeyrie et al. [5] demonstrated permanent aneurysmal retraction rate by 91% by permanent morbidity of 5% due to ischemia and zero mortality. Other studies show persistent occlusion rate >90% and morbidity <5% [6, 7]. Prior to PAO, the BOT should be performed to evaluate the collateral flow in the vessel territory. However, the PAO cannot be performed in cases where the neurological symptoms or venous phase is delayed over 2 s on the angiogram during the occlusion test [8]. Hypotensive challenge during BOT is safe and increases its sensitivity [9].
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
INFORMED CONSENT
Informed consent was obtained from the participant included in this report.
ETHICAL APPROVAL
All procedures performed in this report, involving a human participant, were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. According to local legislation, ethical board review is not mandatory for case reports and retrospective studies.



