-
PDF
- Split View
-
Views
-
Cite
Cite
Gregory Christodoulidis, Athina Α Samara, Konstantinos Perivoliotis, Theodoros Floros, Georgios Volakakis, Dimitrios E Magouliotis, Roidoula Papamichali, Konstantinos Tepetes, Leiomyosarcoma of the spermatic cord presenting as an incarcerated inguinal hernia: a rare presentation of a rare condition, Journal of Surgical Case Reports, Volume 2021, Issue 2, February 2021, rjaa589, https://doi.org/10.1093/jscr/rjaa589
Close - Share Icon Share
Abstract
Leiomyosarcomas of the spermatic cord are rare malignancies with only sporadic cases (less than 150) reported in the literature. Preoperative diagnosis of a paratestical leiomyosarcoma is challenging. Clinicians do not typically consider inguinoscrotal lumps as underlying sarcomas due to their relatively low prevalence compared with hernias. As a result the diagnosis of a sarcoma of the paratesticular area is often hard to reach. Herein, we report a rare case of a leiomyosarcoma originating from the spermatic cord, masquerading as a strangulated inguinal hernia. Intraoperatively, a mass arising from the spermatic cord was found and excised. A supplementary orchiectomy with high ligation of the spermatic cord was also performed.
INTRODUCTION
Spermatic cord and paratesticular tissue sarcomas are a sporadic subtype of neoplasms. These tumours are typically reported as single cases, thus rendering the estimation of their true incidence a challenge [1]. The most common histologic subtypes in adults are liposarcomas, leiomyosarcomas and rhabdomyosarcomas [2]. Leiomyosarcoma accounts for ~10% of all spermatic cord sarcomas in adults and has a peak incidence between the sixth and seventh decade of life. [3]. Spermatic cord leiomyosarcomas are rare malignancies with less than 150 cases reported in the literature [4]. Generally, these tumours present as asymptomatic, painless and slow-growing masses [3].
Herein, we report on a rare case of leiomyosarcoma arising from the spermatic cord, masquerading as a strangulated inguinal hernia.
Case presentation
A 52-year-old Caucasian male was referred to the Emergency Department of our tertiary centre with a painful right inguinal mass. The patient reported that swelling he experienced in the inguinal area had developed over the previous 4 days and that the pain deteriorated with cough and movement. His medical history was unremarkable, with no comorbidities or past operations. Clinical examination revealed a tender palpation, inguinoscrotal protrusion, in addition to signs of cutaneous inflammation. Besides mild leucocytosis, routine laboratory examinations were normal.
Based on the clinical diagnosis of an incarcerated inguinal hernia, the patient underwent an emergency open repair. Intraoperatively, upon opening the inguinal canal, a discrete soft tissue mass was found. The lesion was lobulated and encapsulated a gross cystic component. Dissection validated the absence of any hernia defects and revealed that the mass originated from the spermatic cord (Fig. 1). Frozen section confirmed a neoplastic tissue with mesenchymal origin. Based on this finding, an en-bloc resection of the tumour combined with an orchiectomy and high ligation of the spermatic cord was performed. The patient had an uneventful postoperative recovery and was discharged on the third postoperative day.
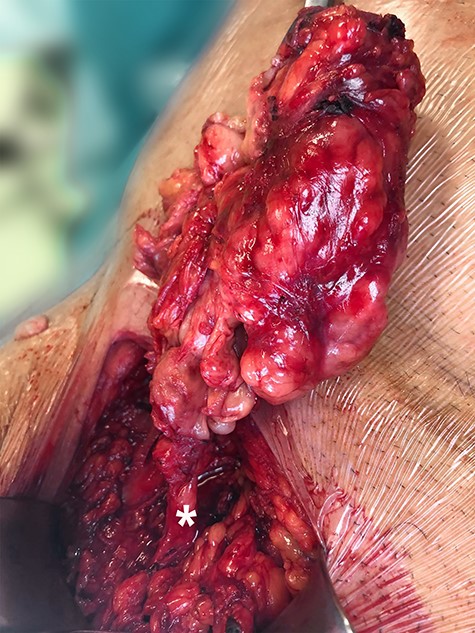
Intraoperative image of the mass, with the asterisk depicting the spermatic cord.
Subsequent staging with a multiple detector computed tomography scan of the abdomen and the thorax did not reveal any distal metastases. The pathology report confirmed the R0 excision of a 10.5 × 9 × 4.5 cm leiomyosarcoma and a yield of 6 negative lymph nodes. A diffuse edematous and myxoid stroma combined with an extensive necrosis (50%) was also noted. The mitotic activity was 11 mitoses/10 HPF (high-power field). Immunohistochemical staining highlighted a positive ΜΙΒ1 in 30–40% of the neoplastic cells. The immunophenotype was caldesmon positive and negative for CKAE1/AE3, SMA, S100, CD34 and c-kit.
A multidisciplinary team discussed the patient’s case and suggested adjuvant chemotherapy and radiotherapy. The patient refused the initiation of the proposed 30 x 2 Gy radiation scheme; he adhered, though, to the proposed chemotherapy protocol with four cycles of gemcitabine 1 g.
DISCUSSION
Hernias are typically the most common diagnosis of inguinal lumps. Οther causes include benign pathologies such as lipomas, cysts and hematomas. However, malignancies, and more specifically mesenchymal tumours, are infrequently associated with inguinal masses. The majority of spermatic cords malignancies have sarcomatous lineage due to the embryologic mesodermal origin; these include leiomyosarcomas, rhabdomyosarcomas, liposarcomas and fibrosarcomas [4]. Leiomyosarcomas are rare malignancies representing ~5–10% of all soft tissue sarcomas [3]. The real prevalence of spermatic cord leiomyosarcomas is difficult to estimate; therefore, only a few sporadic cases have been reported in literature [4].
Preoperative diagnosis of a paratestical leiomyosarcoma is exceptionally difficult and requires a high level of clinical suspicion. Due to their relatively low incidence and misleading clinical presentation, the diagnosis of a leiomyosarcoma is often challenging to reach [5]. Even with the use of imaging studies, the definite differentiation and diagnosis between a benign and a malignant tumour is not typically determined until histological analysis, due to their similar ultrasonographic features [6].
The natural course of a leiomyosarcoma depends on its site, size, grade and nodal or distant metastases [3]. From a pathological point of view, high cellularity, vascular invasion, necrotic areas, presence of multinucleated cells and a mitotic index greater than 2.5 are considered prognostic factors of the biologic behaviour of the tumour [4]. Taking into consideration the large tumour size, the presence of extensive necrotic areas, the high mitotic index (11/HPF) and the presence of multinucleated cells, in our case a poor long-term prognosis was estimated.
The paucity of literature in this area renders therapeutic management a challenge. Radical inguinal orchiectomy with high ligation of the spermatic cord is considered as the gold standard procedure with the longest disease-free survival [2]. Conversely, for small, single and non-infiltrating lesions, a recent multicentre study suggested that conservative surgical resection of the lesion without an en-block orchiectomy could be a feasible therapeutic option [7]. In our patient, based on the intraoperative findings, we considered that the optimal oncological approach should incorporate a radical resection.
In addition, the role of prophylactic lymph node dissection remains unclear [8]. Moreover, due to the rarity of the disease, the lack of randomized trials and the limited experience, the role of adjuvant chemotherapy and radiotherapy remains controversial and is reserved for the presence of metastatic disease [4]. Sarcomas of the spermatic cord have a relative resistance against chemotherapy and in these settings routine adjuvant systemic therapy is not recommended, with the exception of rhabdomyosarcomas [9]. On the other hand, due to the substantial risk of recurrence (up to 50% in certain cases), there is an increased trend on the use of adjuvant chemo and radiotherapy in high-risk patients [1, 8–10]. In any case, a close follow-up including chest X-rays, CT scans and bone scans is necessary [9]. Our patient harboured several negative prognostic features that rendered it as high risk for recurrence. As so, a combined adjuvant scheme was proposed. However, without a gold-standard treatment of these patients the individualization of each case is necessary.
Malignant tumours of the spermatic cord are typically slow-growing, painless lumps in the inguinal area. In our case, the extensive necrotic cystic component, resulted in symptoms compatible with an incarcerated inguinal hernia. Only a small number of spermatic cord leiomyosarcomas presenting with acute symptoms have been reported in the literature, thus confirming the rarity of this clinical event [10].
Leiomyosarcoma of the spermatic cord should be included in the differential diagnosis of elderly males presenting with an inguinal mass, even in patients with symptoms typical of an inguinal hernia.
CONFLICT OF INTEREST
None declared.
FUNDING
None.



