-
PDF
- Split View
-
Views
-
Cite
Cite
Christoph Kuemmerli, Patricia Sánchez-Velázquez, Christoph Tschuor, Christian Oberkofler, Mario Lachat, Beat Müllhaupt, Pierre-Alain Clavien, Henrik Petrowsky, When Echinococcus granulosus transmigrates from the liver into the pericardium: a case report, Journal of Surgical Case Reports, Volume 2021, Issue 2, February 2021, rjaa492, https://doi.org/10.1093/jscr/rjaa492
Close - Share Icon Share
Abstract
Infection with Echinococcus granulosus is a common helminthic disease worldwide with endemic in a region with high endemic areas in Africa, Asia, Middle East, South America and southern Europe.
We report a rare case of a young patient with cystic echinococcal disease of the liver invading the pericardium. The patient initially presented with life-threatening cardiac tamponade, which resulted in the discovery of the underlying parasitic disease. He successfully underwent en-bloc hepatic pericystectomy and pericardiac resection with closure of the pericardial defect using a xenogeneic patch. After this procedure, he recovered well and had no cardiac complications in the long term. Under treatment with albendazol, the patient showed no signs of recurrent disease.
Cases of complex cystic echinococcosis, which invade adjacent organs or body cavities, often need radical surgery for definitive treatment embedded in a multidisciplinary approach in highly specialized centers.
INTRODUCTION
Infection with Echinococcus granulosus is a common helminthic disease worldwide with high endemic areas in Africa, Asia, Middle East, South America and southern Europe [1]. Due to the predominant liver affection, upper abdominal complaints are the most common symptoms, which often lead to the detection of the disease, although the presentation might vary depending on the distribution of organ involvement [2, 3]. Current treatments include antihelminthic medication, conservative and radical procedures.
CASE REPORT
A 29-year-old otherwise healthy patient born in southern Europe was referred to our surgical intensive care unit after presenting with two syncopal episodes secondary to cardiac tamponade. Apart from epigastric tenderness, the physical examination was unremarkable. Liver function tests and renal function were within the normal range. Magnetic resonance imaging revealed a well-defined, multiloculated cystic lesion in the left liver with internal hyperdense septations, probably infiltrating the pericardium with floating cysts and a moderate pericardial effusion (Fig. 1A). Because of hemodynamic stability, including low central venous pressure, pericardial drainage was dispensed and the patient was transferred to the surgical ward. Due to the suspected diagnosis of echinococcal disease, empiric treatment with albendazole was started. Serology tests by enzyme-linked immunosorbent assay confirmed parasitic infection with E. granulosus. The imaging features are consistent with stage CE2 of the World Health Organization (WHO) classification system for cystic echinococcosis [4].
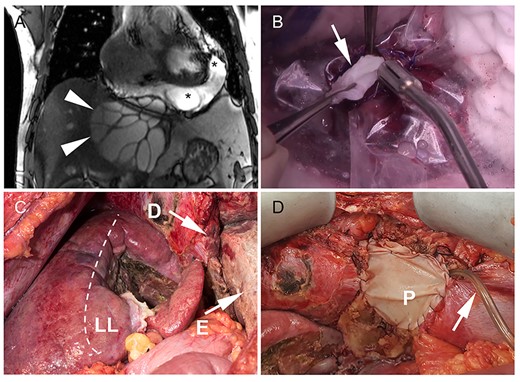
Cystic echinococcosis of the liver with pericardial involvement. (A) On magnetic resonance imaging, arrowheads indicate the right border of the cyst. Liver segments I–IV are atrophic due to the compression of the cyst (diameter 13.4 cm). Daughter cysts are detected in the pericardial effusion (asterisk); (B) to avoid dissemination and spilling of the cyst content, a plastic cover was sutured to the cystic wall before opening of the cyst. The parasitic cysts (arrow) were removed; (C) intraoperative situs after completed pericystectomy. The dashed line marks the anatomic border between segments V/VIII and IV. The diaphragm was infiltrated by the parasitic cyst (D) and resected with adjacent pericardium. The image shows the open pericardium with severe fibrinous pericarditis (E); (D) pericardial defect (P) was closed with a xenogeneic patch and a pericardial drain (arrow) was placed for postoperative irrigation with hypertonic saline solution (D).
Scheduled surgical exploration revealed a large cystic mass arising from the left liver lobe with adherence to the diaphragm. The cystic lesion was dissected from the liver and suprahepatic vena cava using water-jet dissection. After complete hepatic dissection, the diaphragmatic part of the pericardium showed parasitic erosion with transpericardial invasion of echinococcal cysts. Resection of the pericardium was necessary to completely evacuate the echinococcus cysts. First, hypertonic saline solution (25%) was injected into the cyst while carefully avoiding intraabdominal spillage. Following incision of the pericardium (Fig. 1B), severe fibrinous pericarditis was found, multiple cysts were evacuated and the pericardium was debrided. Finally, en-bloc resection of the extrapericardial cyst was then completed (Fig. 1C). The pericardial defect was closed with a xenogeneic pericardial patch and a transpericardial drain was placed for postoperative irrigation with hypertonic saline solution. Planned second-look surgery was performed at the first postoperative day. Inspection showed a thrombosed greater omentum and persistent fibrinous pericarditis. The greater omentum and the fibrinous coating of the pericardium were resected as well as debrided. The pericardial defect was closed again with a patch (Fig. 1D). Histology confirmed cystic echinococcosis with pericardial transmigration (Fig. 2A and B).
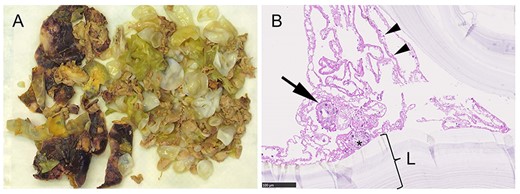
Macro- and microscopic specimens. (A) Resected cyst material consists of the darker cyst wall (white asterisk) and the yellow and white daughter cysts; (B) histology revealed multiple protoscoleces (black asterisk), one of them with a hook (arrow). The arrowheads point at the germline membrane, which corresponds to the inner layer of the cyst. The laminated membrane (L) is surrounding the germline membrane. The outermost layer, the pericyst, is not shown. It consists mainly of host cells.
The patient developed supraventricular tachycardia most likely associated with the pericardial manipulation and rate control was achieved under amiodarone. He was discharged on postoperative day 15. The day after discharge, the patient was readmitted due to a fascial dehiscence, which required fascial closure with mesh augmentation. Three months after surgery, a hemodynamically relevant pericardial infusion was punctured. Another 22 months later, the patient underwent laparoscopic diaphragm for symptomatic enterothorax. Ultrasonography showed no signs of recurrence. Medical treatment with albendazol was continued for the time being.
DISCUSSION
Cystic echinococcosis is a common helminthic zoonosis in the Mediterranean area caused by E. granulosus [1]. In the intermediate host, like humans, the echinococcus oncocospheres developing from parasitic eggs cross the intestinal wall and transform into larvae, which are then carried out via portal vein flow into the liver and other organs. There, larvae undergo a metamorphosis towards the metacestodes. The metacestodes implant into the organ and grow into cysts. The echinococcal cyst consists of periparasitic host tissue, the pericyst or adventitia surrounding the endocyst. The latter is composed of an outer, acellular laminated membrane and an inner layer, the germinal membrane [5]. Protoscoleces arise from the germinal layer. Further development into adult tapeworms is only possible in a definitive host such as dogs and other Canidae [6]. Some cysts may contain daughter cysts.
The cysts are typically located in the liver, although this infection has been described in almost every organ in the human body. They usually show repressive growth respecting anatomical borders in contrast to infection with Echinococcus multilocularis. Infiltrative growth into adjacent structures or organs is rarely reported in cystic echinococcosis [7, 8]. Multiple organ involvement is rare and disseminated disease is a scarcity. The involvement of the heart, as presented in our case, is reported in about 2% of cases and out of these less than a quarter show pericardial involvement [9].
The most fatal complication in cystic echinococcosis is cystic rupture with spillage of the cyst content, followed by septic complications and life-threatening anaphylaxis. Although this is also a possible jeopardy in pericardial or myocardial involvement, the mass effect of the cyst or a reactive pericardial effusion and consecutive cardiac tamponade can, at this particular localization, be more detrimental and life threatening, as shown in our case.
Treatment of abdominal cysts is determined by the cyst stage. The classification of the cyst stage is based on the five-stage WHO classification grading system (CE1–CE5), which is determined by features of cyst composition and cyst wall [4]. Depending on cyst stage, drugs, mostly albendazole or mebendazole, are used either alone or in combination with surgery or interventional procedures such as, e.g. puncture, aspiration, instillation, reaspiration (PAIR). Drugs are usually given between 1 day and 1 week before surgery and between 1 and 3 months after the intervention. However, the optimal duration of medical treatment either before or after the intervention are poorly defined. Based on the two-cavity involvement with spread of cysts in the liver and pericardium in the presented case, we aim at this time a long-term treatment with albendazole after surgery.
Among surgical therapies, conservative and radical procedures are available. Radical surgery focuses on the complete removal of the parasitic material, the most effective treatment with the lowest recurrence rate. In contrast, the nonoperative approach addresses only the cyst content leaving the cyst wall. Surgery is the standard of care in complicated cysts, which includes large CE2–CE3b cysts with daughter vesicles, as present in our case, cysts at risk of rupture, infected cysts and cysts communicating with the biliary tree. In addition, cysts exerting pressure on adjacent vital organs are another indication for surgery. For large CE1 and CE3a cysts in inoperable patients and those who refuse surgery, the percutaneous PAIR approach provides a less invasive treatment option [4].
The reported case is notable in the way it illustrates the potential life-threatening complications in a rarely affected organ in complex cystic echinococcosis. In such cases, surgical treatment remains the only curative treatment. The therapy may need highly specialized centers and a multidisciplinary approach.
INFORMED CONSENT STATEMENT
The patient gave written consent to publish the case report.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
References
- pericardial sac
- cardiac tamponade
- liver diseases
- congenital pericardial defect
- africa
- asia
- cysts
- helminthiasis
- middle east
- parasitic diseases
- south america
- surgical procedures, operative
- infections
- liver
- surgery specialty
- echinococcus granulosus
- echinococcus granulosus infections
- cardiac complications



