-
PDF
- Split View
-
Views
-
Cite
Cite
Danielle Abbitt, Abigail L Barnes, Hazem T Hammad, R Matthew Reveille, Edward L Jones, Endoluminal vacuum closure of a duodenal perforation, Journal of Surgical Case Reports, Volume 2021, Issue 11, November 2021, rjab479, https://doi.org/10.1093/jscr/rjab479
Close - Share Icon Share
Abstract
Perforation is a known complication of endoscopic resection and has been managed with endoscopic defect closure, antibiotics and close observation. Closure of duodenal perforations are more challenging due to the presence of gastric and pancreaticobiliary secretions. The use of endoluminal vacuum therapy (EVT) to divert flow and aid closure is increasingly prevalent and may avoid high-risk surgery. We describe the use of endoluminal vacuum closure to salvage an iatrogenic duodenal perforation in a 57-year-old male who underwent an endoscopic mucosal resection of a 35-mm polypoid lesion on the posterior wall of the second portion of the duodenum. The endoluminal wound vac successfully controlled leakage and allowed defect closure. EVT is an emerging technique that can effectively manage complicated injuries throughout the GI tract and may allow enhanced recovery by avoiding surgical salvage and its associated morbidity and mortality.
INTRODUCTION
Perforation is a known complication of endoscopic mucosal resection (EMR) and has been managed with endoscopic defect closure with endoclips or suturing, antibiotics and observation. EMR is less often performed for duodenal lesions due to concerns for perforation secondary to a narrow duodenal lumen, sharp flexures that decrease endoscope stability and duodenal wall stiffening [1, 2]. Closure of a duodenal perforation is more challenging due to gastric and pancreaticobiliary secretions. Tissues are friable after exposure to these secretions making further clipping or suturing difficult if not immediately addressed.
If a perforation is identified at the time of the procedure, it can be treated with endoscopic clipping. Endoscopic suturing is an emerging option for immediate and delayed defect closure but additional training and specialized equipment are required, thus not widely offered [3]. Therefore, the use of endoluminal vacuum therapy (EVT) to divert flow and aid closure of a luminal perforation has increased and efficacy demonstrated throughout the gastrointestinal (GI) tract.
We present a case where endoscopic and surgical attempts at closure of an iatrogenic duodenal perforation failed and radical resection avoided by successful EVT.
CASE REPORT
A 57-year-old male underwent EMR of a 35-mm polypoid lesion on the posterior wall of the second portion of the duodenum (Fig. 1a and b). This was complicated by intra-procedural bleeding (250 ml), which was stopped with epinephrine injection and Coagrasper (Olympus America, Center Valley, PA) application. The mucosal defect was closed with 12 endoclips (QuickClip Pro, Olympus America, Center Valley, PA). He developed evidence of perforation on post-procedure Day 1 (Fig. 1c and d) and was taken to surgery, the area irrigated and widely drained. Closure was not attempted due to severe thickening and friability of the duodenum.
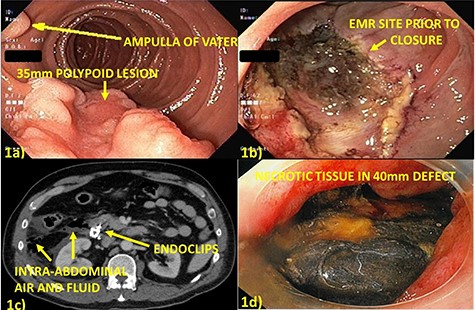
(a) Thirty-five millimeters polypoid lesion on posterior wall of second portion of the duodenum, adjacent to the ampulla of Vater; (b) Mucosal defect after EMR of the lesion; (c) Computed tomography post-procedure Day 1 with free, intraperitoneal air and fluid adjacent in the right paracolic gutter and retroperitoneum suggesting perforation; (d) Endoscopy post-procedure Day 6 with 40 mm defect at site of prior closure, bile-stained necrotic tissue overlying retroperitoneum and free mucosal edges.
The patient clinically worsened over the next 48 h. Repeat imaging demonstrated uncontrolled leakage, which was managed with radiographically-placed drains. He continued to deteriorate, which promoted discussion of urgent pancreaticoduodenectomy; however, his tenuous situation predicted poor operative outcomes. EVT was placed on post-procedure Day 6 as an attempt to control duodenal leakage and prevent surgical re-exploration and salvage pancreaticoduodenectomy.
The endoluminal wound vacuum device was created with an 18Fr nasogastric tube (NGT), placed via the nare and withdrawn through the oral cavity where it was cut at the most proximal port (Fig. 2a). A piece of black sponge (VAC® GRANUFOAM™, KCI USA, San Antonio, TX) was cut to ~15 mm in diameter and sutured to the NGT with two, 0-Ethibond sutures. Both sutures included a generous piece of the black sponge and traversed the lumen of the NGT to avoid unintentional dislodgement during placement or retrieval. This was placed into the defect under direct visualization and suction (125 mmHg) initiated (VAC® ULTRA™, KCI USA, San Antonio, TX). The endoscope was withdrawn taking care not to dislodge the sponge (Fig. 2b).
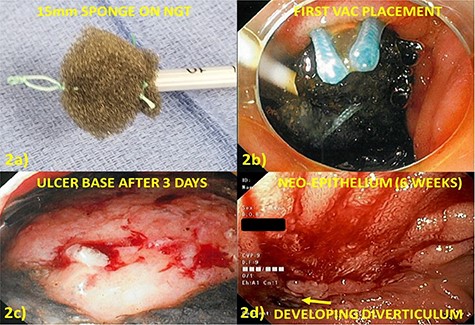
(a) Endoluminal vacuum closure sponge created by suturing a 15-mm piece of black sponge to an 18fr NGT with 0-ethibond suture. A loop is created to aid in manipulation and placement at the tip of the sponge. (b) Cap-aided placement of sponge in defect which is held in place by activating suction. (c) Debridement of retroperitoneal necrosis at first exchange (3 days after placement). (d) Defect appearance at 6 weeks after cessation of therapy with neo-epithelium covering nearly the entire defect.
The sponge was exchanged every 3–6 days depending on drain output and ability to maintain suction. The WV was exchanged six times over 31 days and was removed when there was evidence of circumferential adherence of the duodenum to the retroperitoneal and no drainage from nearby drain. EVT successfully controlled gastric and pancreaticobiliary secretions allowing defect closure and avoidance of highly morbid surgical re-exploration (Fig. 2c and d). The final pathology confirmed a tubular adenoma with focal high-grade dysplasia and clear margins. Surveillance EGD at 12 months demonstrated complete closure of defect with mild scarring and no evidence of residual or recurrent adenoma (Fig. 3a and b).
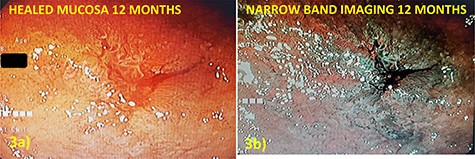
(a) Defect appearance at 12 months on white light and (b) narrow-band imaging without evidence of recurrence or other abnormality.
DISCUSSION
EVT was first described in 2007 for the closure of rectal and esophageal leaks with use now in nearly all portions of the GI tract [4, 5]. The success of EVT is a result of (i) providing continuous drainage to the wound thereby reducing damage to surrounding structures from intraluminal secretions; (ii) increasing the formation of granulation tissue and (iii) increasing vascularity in the area of the sponge [6].
Since its initial description, there has been a dramatic increase in the use of EVT in the realm of esophago-gastric leaks and perforations [7]. Several publications describe successful defect closure in the esophagus, stomach and rectum, with decreased need for surgical repair [8–11] and reduced mortality to 12% from 50% in patients treated surgically or 83% in patients treated with stent [12].
There are few publications on the use of EVT in small bowel. The largest case series included 11 patients with a duodenal perforation, secondary to perforated ulcer, stricturoplasty, gastric resection or endoscopic retrograde cholangiopancreatography [13]. Closure required a median 11 days (range 7–24) and sponges were exchanged every 2–7 days. All (100%) of these patients had successful closure of their defects via EVT.
The greatest disadvantage is the need for repeated endoscopic procedures for sponge changes. This requires personnel who are trained, available and have access to endoscopy suites or the OR for the entire duration of therapy. The most worrisome complication of EVT is hemorrhage secondary to erosion into nearby, major vessels [14]. Frequent sponge changes may reduce this risk by reducing tissue in-growth into the sponge as well as allowing frequent examination of the lesion. Pre-EVT review of cross-sectional imaging is mandatory to avoid placement of the sponge directly on exposed major vasculature.
When considering gastro-esophageal leaks, the most common indication for EVT, the previous standard of care was self-expanding metal stents (SEMS). Nearly all recent data confirm the superiority of EVT over SEMS with the most recent review by Jung et al. demonstrating an 85% overall success rate when compared to SEMS [15]. The authors also reported a significantly lower mortality and duration of therapy. This observation is not surprising given the typical duration of treatment with SEMS (4–6 weeks before exchange) in contrast to the frequent re-examinations every 3–5 days with EVT.
In summary, EVT is an emerging technique that can salvage complicated perforations, with this case and the above references confirming the effectiveness and safety of EVT in the peri-ampullary region. This important technique should be considered in all patients who suffer a GI tract perforation or leak that can be reached endoscopically.


