-
PDF
- Split View
-
Views
-
Cite
Cite
Seyed Amir Miratashi Yazdi, Pedram Habibi, Arezoo Eftekhar Javadi, Reza Hajebi, GIST case turned out to be a perforated peptic ulcer: a case report, Journal of Surgical Case Reports, Volume 2021, Issue 10, October 2021, rjab494, https://doi.org/10.1093/jscr/rjab494
Close - Share Icon Share
Abstract
Gastrointestinal stromal tumors (GISTs) are tumors arising from the fourth layer of the stomach. They are the most common form of mesenchymal neoplasms in the gastrointestinal tract. The diagnosis for the aforementioned tumors is made through endoscopic ultrasonography (EUS) with no further inspection through biopsies and aspirations. Regarding the fact that biopsies are not made in these cases, pathological misdiagnoses, however rare and effectless in the final outcome of the surgery, do occur. Here, we present a case of a 35-year-old male diagnosed with GIST through the means of EUS however, the post-op pathology report showed something very interesting. This patient was reported to be a case of perforated peptic ulcer and we found vegetable fiber component in the tissue of this patient. Bearing in mind the finding, misdiagnoses of GIST patients are probable, and careful planning prior to the surgery is recommended.
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are believed to arise from cells of Cajal, which are also known as the pacemakers in gastrointestinal (GI) movements [1]. They are the most common type of mesenchymal neoplasms in the GI tract [2]. Between 10 and 30% of GISTs become malignant [3].
GIST diagnosis is mostly accomplished through endoscopic ultrasonography (EUS) [4] with no need for biopsy due to the risk of bleeding and the consideration that often biopsies results are non-representative due to the submucosal position of GISTs.
Surgery holds the place for the best modality regarding permanent cure of the tumor. The surgery is aimed toward full resection of the tumor with as much preserved functionality as the situation allows. The role of margin status is not well defined. It has been suggested before that it may even have no effect on the prognosis of the patient [5].
Seldom EUS reports mistake other pathologies with GIST. PPU is a medical emergency with a mortality rate of ~30%. The most common causes of PPU are Helicobacter pylori and use of non-steroidal anti-inflammatory drugs [6].
CASE REPORT
A 35-year-old male referred to the hospital with abdominal pain and intermittent food intolerance, concomitant with 6 kg of weight loss since 3 months earlier. No prior history of GI disease, drug use, abdominal pain and similar problems in his family were observed. In the abdominal examination, the abdomen appeared to be normal and no distension or organomegaly was observed. Also, no tenderness, rebound tenderness and guarding were noted. Prior to referral to the surgery clinic, the patient had undergone endoscopy and computed tomography (CT) scan, and the reports are as follows.
Endoscopy report
Stomach: normal fundus, normal body and a large submucosal mass lesion with partial obstruction of the gastric outlet.
Duodenum: Bulbar deformity, normal D2.
CT scan report
Increased gastric wall thickness was observed with emphasis on antropyloric region and the lesser curvature with maximum thickness of 21 mm accompanied by fat stranding around the same areas. Evidence of partial stenosis with mild dilatation of stomach was seen. Contrast agent has penetrated through the distal region and no leak of contrast agent is observed. Liver, spleen and the pancreas looked normal. No apparent paraaortic lymphadenopathy was observed. No free fluid was observed in the abdomen and the pelvic cavity (Figs 1 and 2).
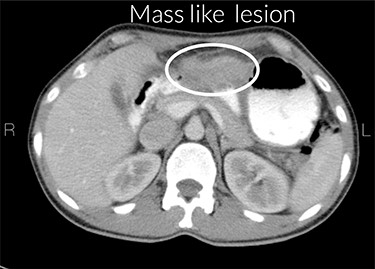
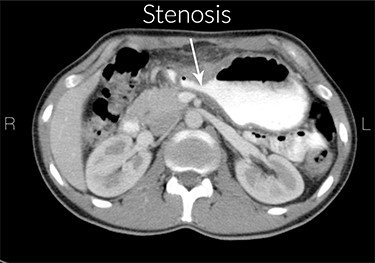
Stenosis of the gastric outlet. R means right and L means left.
Considering the aforementioned reports, EUS was requested, and the report was as follows: two large sub epithelial lesions were seen in the antral territory that caused partial obstruction of gastric passage.
Upper GI: There was large mixed echo mass 3244 mm in size with marked border with three cystic lesions in it and another one, a 26 × 22 mm SEL, both of which originated from forth layer of the gastric wall. The lesion is located between the left liver lobe and the outlet of the stomach. One lymph node was seen around the lesions.
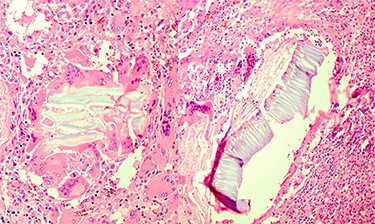
Multinucleated foreign body-type giant cells and inflammatory cells surrounding the vegetable fibers.
Hepatobiliary: At visible range, there was no intrahepatic ducts dilation, with no intrahepatic masses or lesions.
Final diagnosis: GIST with worrisome signs is a highly probable diagnosis.
LAB FINDINGS
At laparotomy, severe adhesions of the omentum, falciform ligament, and left hepatic lobe to the stomach antrum were noted, below which a mass like lesion was felt and observed. The stomach was deformed and dilated, with no evidence of seeding or metastasis in the peritoneal cavity. Separation of the stomach and the liver was not feasible. On this patient, distal gastrectomy, resection of Segment II of the liver en bloc with the stomach and Billroth II were performed. Five days after the surgery, the patient was discharged with stable general condition and food tolerance.
| WBC: 9.1 . | RBC: 3.5 . | Hb: 9.8 . | Plt: 367 . | Cr: 1.2 . | Na: 144 . | K: 4.3 . |
|---|---|---|---|---|---|---|
| AST: 23 | ALT: 12 | ALP: 165 | PT: 13.7 | PTT: 41 | INR: 1.08 |
| WBC: 9.1 . | RBC: 3.5 . | Hb: 9.8 . | Plt: 367 . | Cr: 1.2 . | Na: 144 . | K: 4.3 . |
|---|---|---|---|---|---|---|
| AST: 23 | ALT: 12 | ALP: 165 | PT: 13.7 | PTT: 41 | INR: 1.08 |
| WBC: 9.1 . | RBC: 3.5 . | Hb: 9.8 . | Plt: 367 . | Cr: 1.2 . | Na: 144 . | K: 4.3 . |
|---|---|---|---|---|---|---|
| AST: 23 | ALT: 12 | ALP: 165 | PT: 13.7 | PTT: 41 | INR: 1.08 |
| WBC: 9.1 . | RBC: 3.5 . | Hb: 9.8 . | Plt: 367 . | Cr: 1.2 . | Na: 144 . | K: 4.3 . |
|---|---|---|---|---|---|---|
| AST: 23 | ALT: 12 | ALP: 165 | PT: 13.7 | PTT: 41 | INR: 1.08 |
Pathology report
Portions of the gastric wall with acute and chronic inflammation were received. Fat necrosis, foreign body-type giant cells, granulomatous formation and fibroblastic proliferation in serosal and perigastric fat were observed, which had infiltrated submucosa in some areas and were attached to the liver tissue (vegetable fibers component?).
Perigastric fat tissue also shows acute and chronic inflammation, fat necrosis and foreign body-type granulomatous formation.
Omentum shows focal foreign body-type granulomatous formation (Fig. 3).
DISCUSSION
Peptic ulcer disease has been shown to be related with urbanization [7]. Complications might occur following this disease, such as perforation, obstruction, bleeding and malignancy. Perforated peptic ulcer (PPU) is associated with high rates of mortality: around 30%. Considering the circumstances, perforation of a peptic ulcer requires immediate attention and is considered to be a medical/surgical emergency.
GISTs have been shown to arise from the fourth layer of the stomach [1]. Ten to 30% of GISTs become malignant [3]. The diagnosis of GIST is accomplished through EUS with no further aspiration and/or biopsy, bearing in mind the high bleeding risk of these tumors.
For our patient, after endoscopy and CT scan, EUS was proposed as the final pre-surgical diagnosis, and the report suggested GIST to be the pathology of the tumor. The patient denied any prior similar pain in the past and was transferred to the operating room as a case of GIST. In the operating room, a mass-like lesion was observed and removed. However, it was the pathology report that made this case interesting enough to be included as a case report. The report came out as vegetable fibers component inside the received tissue. Our suspicions are that after the peptic ulcer formed a perforation, food material exited the GI tract and grew to be a vegetable fiber component.
Regarding our findings, a peptic ulcer that is walled off with the omentum could potentially mimic a tumor and pre-op paraclinicals might not be as accurate as one might suspect.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
The authors have received no funding from any institutions.
INFORMED CONSENT
The patient understood that his clinical data would be used for publication purposes and provided his consent.



