-
PDF
- Split View
-
Views
-
Cite
Cite
Michele R Colonna, Alfio L Costa, Claudio Mastrojeni, Vincenzo Rizzo, Giuseppe Nirta, Filippo F Angileri, Antonio Ieni, Erica Milone, Antonio Macrì, Giant sacral schwannoma excised under intraoperative neuromonitoring in an elderly patient: case report, Journal of Surgical Case Reports, Volume 2021, Issue 10, October 2021, rjab460, https://doi.org/10.1093/jscr/rjab460
Close - Share Icon Share
Abstract
Schwannomas are mainly benign tumors arising from the Schwann cells of the peripheral nerve sheath. These tumors can often be associated with non-specific symptoms, such as abdominal heaviness. In this article, we present a detailed description of the surgical management of a giant sacral schwannoma in an elderly patient, for which intraoperative neuromonitoring made it possible to distinguish easily the nerves of the sacral plexus from which the tumor originated and to remove it without complications. Treatment of these rare and symptomatic giant tumors is still a challenge for surgeons; to treat adequately these tumors; a multidisciplinary approach is required to ensure an optimal therapeutic approach to reduce the risk of recurrence and, on the other hand, is not associated with unnecessary iatrogenic neurological damage.
INTRODUCTION
Schwannomas are mainly benign tumors arising from the Schwann cells of the peripheral nerve sheath. These tumors usually consist of well-differentiated cells [1, 2]. They can originate de novo or in the context of genetic conditions such as Type 2 neurofibromatosis [3]. These tumors can grow anywhere in myelinated fibers of the peripheral nerve, but in some areas the finding is more frequent than in others. At the spinal level, they are relatively frequent, especially in the thoracic region, and represent 25% of all primary spinal tumors [4]. The sacral localization, on the other hand, is quite rare and represents only 1–5% of spinal schwannomas [5]. Generally, these tumors do not infiltrate the nerves themselves and remain separate in the nerve sheath. The slow growth of these tumors can lead, in the sacral area, to a dislocation of the nerves and viscera, before giving a compressive symptomatology with dysesthesia and lumbar pain [6–9]. Therefore, sacral schwannomas can reach considerable size before being diagnosed usually on magnetic resonance imaging (MRI). The treatment of these rare and symptomatic giant tumors is a challenge for the surgeon; surgical excision with curative intent must solve the symptoms and avoid relapses, on the other hand, sparing as much as possible the peripheral nerves from which the tumor originates [10]. In this article, we present the detailed description of the surgical management of a giant sacral schwannoma in an elderly patient, for which intraoperative neuromonitoring made it possible to easily distinguish the sacral plexus nerves from which the tumor originated and to remove it without complications.
CASE DESCRIPTION
History and preoperative investigations
A 62-year-old male presented to our department with a history of abdominal heaviness and dysesthesia in the lower limbs for ~6 months. A computed tomography scan showing an expansive mass needing further investigation through MRI. MRI highlighted, in the pelvic excavation, a paramedian, well-defined heterogeneous lesion of mixed-signal intensity of ~115 × 105 × 92 mm with polylobulate margins and multiple intralesional septa (Fig. 1), which is characterized by heterogeneous hyper-intensity of signal in the T2-weighted sequences and hypo-intensity in T1. An ultrasound-guided biopsy was suggestive for schwannoma.
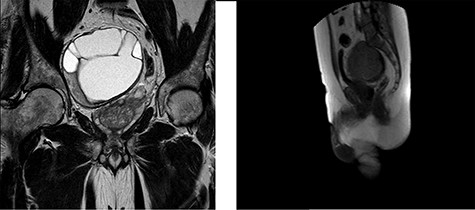
MRI of the pelvis in the Coronal Plane (left) and Sagittal Plane (right) demonstrating a 115 × 105 × 92-mm sacral schwannoma, displacing the prostate and rectum to the left side.
Surgical technique combined with neuromonitoring
The patient underwent a surgical resection through a single stage procedure in combination with neuromonitoring. The neuromonitoring paradigm consisted of transcranial motor evoked potential (TCeMEPs), as well as spontaneous EMG (free-run EMG) recorded from the external anal sphincter (EAS), bulbocavernosus (BULB) and lower extremity muscles bilaterally [11]. Moreover, the lumbosacral plexus and the pudendal nerve were monitored by direct current stimulation of the nerves. Medtronic E4 NIM Eclipse neuromonitoring system (Medtronic, Minneapolis, MN) was used for neuromonitoring.
TCeMEP was obtained through transcranial electrical stimulation, C1–C2 and reverse (SI 10/10) with train stimulus of 7 impulses, 50-μs duration, ISI 2 ms. Signals recording through subcutaneous needles inserted in the following muscles: maximus gluteus (MG), rectus femoris (RF), tibialis anterior, gastrocnemius medial head, EAS, BULB. free-run electromyography (free-run EMG) was continuously recorded from the nerve roots deriving from the above-mentioned muscles for the TCeMEP. Finally, a bipolar probe was used for monitoring the lumbosacral plexus. The currents applied ranged from 5 to 10 mA, the frequency was 2 Hz, and stimulation lasted 5–20 s.
Surgical access to the giant schwannoma was achieved through an anterior approach (Figs 2 and 3). During the surgery, the stimulation of the right lumbosacral plexus, with bipolar probe, allowed the identification and sparing of the nerves. During the surgical exeresis, no significant variations of the electrophysiological context were observed.
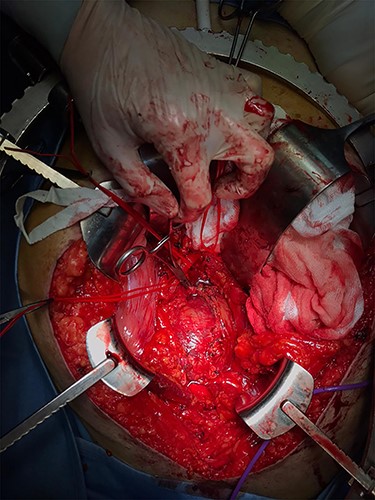
Intraoperative image of surgical resection through a supraumbilical-pubic laparotomy (anterior approach) showing the absence of a clear cleavage plane.
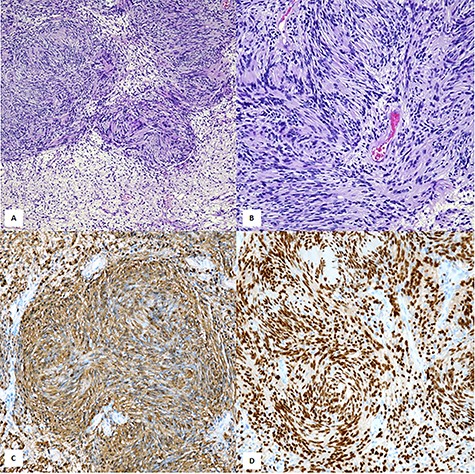
Histopathological examination showed a neoplastic proliferation composed of cellular Antoni A areas alternating with hypocellular Antoni B areas (A,×100, H&E). In cellular areas were encountered the so-called ‘Verocay bodies’ represented by a highly ordered arrangement of Schwann elements separated by fibrillary processes (B, ×200, H&E). Diffuse cytoplasmic immunostaining with S-100 (C, ×200, Mayer’s hemalum counterstain). Strong nuclear positivity with SOX-10 (D, ×200, SOX-10 immunohistochemistry).
Postoperative course
The postoperative course showed a mild strength deficit in the right lower limb: dorsiflexion of the right foot (4/5), abduction of the thigh (4/5) and distal anesthesia L5–S1 right. The symptomatological set described showed a rapid and progressive improvement already in the first postoperative days. Electromyography examinations at 1 and 7 months confirmed the progressive functional recovery.
DISCUSSION
Giant sacral schwannomas are very rare and only case reports or case series are reported in the literature. Klimo et al. classified these tumors based on relationships to the sacrum [12]. Type III tumors, according to this classification, limited to the pre-sacral space, are among the rarest and the present case is one of the largest 115 × 105 × 92 mm in a male subject in the seventh decade of life. These data differ from the literature, which reports a higher frequency in females and in the third to fifth decade [5, 13].
For these tumors, the curative treatment is surgical excision. Since these are benign tumors, one might think that there is a clear cleavage plane, but due to the slow growth and initially asymptomatic course, this tumor is associated with firm adhesions to the abdominal nerves and viscera [9]. In fact, the literature reports a high postoperative morbidity and specifically a neurological deficit in 21% of cases [8].
Regarding the extent of the resection, in some case reports a radical approach is presented, associated with a high incidence of complications [6], whereas in other series of cases partial excision is used, but in some reports it is associated with a higher incidence of relapse [6, 13]. In case of recurrence, postoperative morbidity is much greater due to secondary adhesions to the first surgery; in addition, in case of relapse, the risk of malignant transformation increases [14]. The recent implementation of intraoperative neuromonitoring seems to be associated with a complications rate reduction [4, 8, 9, 11–13, 15]. In our experience, this has allowed an easier discernment of functional nervous tissue, allowing an en-block removal without long-term complications, but only a transient deficit with a rapid functional recovery, even in an elderly patient. For this reason, we found it useful to describe extensively the intraoperative neuromonitoring procedure. Neuromonitoring also facilitates surgical removal of the tumor mass by the surgeon, should reduce the risk of recurrence reported in the literature (16% in case series of Pongsthorn et al.) [13], and associated with an increased risk of malignant transformation. From our point of view, the latter point represents a priority as well as avoiding unnecessary neurological deficits.
CONFLICT OF INTEREST STATEMENT
The author (s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
Author notes
M. R. Colonna and A. L. Costa contributed equally to this work.



