-
PDF
- Split View
-
Views
-
Cite
Cite
Akeel M Merchant, Mark D Friedland, Marcus A Behrens, Adam R Williams, Andrew Berchuck, Nicole R Guinn, Surgical management of ovarian carcinosarcoma with inferior vena cava extension into the right atrium, Journal of Surgical Case Reports, Volume 2020, Issue 12, December 2020, rjaa530, https://doi.org/10.1093/jscr/rjaa530
Close - Share Icon Share
Abstract
Gynecological carcinosarcomas are aggressive tumors with rare occurrence and high rates of metastases. We present the case of a 49-year-old woman with vaginal bleeding and abdominal distension who was found to have a large ovarian carcinosarcoma invading the gonadal vein and inferior vena cava (IVC) and extending into right atrium (RA). She underwent gynecologic tumor resection, IVC cavotomy and en bloc resection of tumor/thrombus through the RA. Use of intraoperative transesophageal echocardiography helped assess extent and mobility of mass in the RA to guide surgical approach. This case posed unique challenges with regard to management of induction, hemodynamics and coagulopathy.
INTRODUCTION
Gynecological carcinosarcomas (GCS), which were previously termed malignant mixed Mullerian tumors, are rare highly aggressive cancers. Uterus and ovaries are the common sites of origin, and other sites include the cervix, vagina and fallopian tubes [1]. Ovarian carcinosarcomas account for only ~1–4% of all ovarian malignancies in the USA [2]. Carcinosarcomas are made up of epithelial (carcinomatous) and mesenchymal (sarcomatous) tissues [3]. Due to their aggressive nature, they have a high rate of metastases and venous thromboembolism (VTE) due to tumor/thrombus extending from the gonadal vein into the inferior vena cava (IVC). While the most common sites of metastasis are peritoneum, lymph nodes and lungs [4], tumor extension into the heart has been described once in the literature [5]. In this report, we present the case of a patient with a large ovarian GCS with IVC extension into the right atrium (RA). A Health Insurance Portability and Accountability Act (HIPAA) authorization was obtained from the patient for publication of this case report.
CASE REPORT
A 49-year-old perimenopausal African American female presented to an outside hospital emergency department (ED) with a 1-month history of intermittent vaginal bleeding, abdominal pain, bloating, constipation and unintentional weight loss. She was found to have a 22-cm pelvic mass on computed tomography (CT) scan, elevated CA-125 and significant anemia requiring blood transfusion and a negative endometrial biopsy. She was scheduled for an elective exploratory laparotomy but re-presented to the ED with worsening abdominal pain, nausea and vomiting prior to her surgery. Repeat CT scan showed interval increase in size of pelvic mass to 27 cm (Fig. 1), abdominal ascites, newly occlusive IVC tumor/thrombus extending into the RA and segmental pulmonary embolism (PE) in the left lower lobe pulmonary artery branches. She was transferred to our hospital for further management given her case complexity.
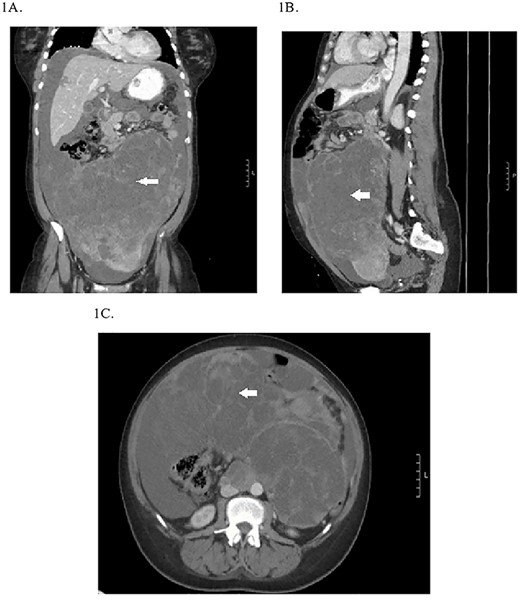
Preoperative CT abdomen/pelvis showing the pelvic tumor in coronal (A), sagittal (B) and transverse planes (C).
Preoperatively, the patient required nasogastric tube (NGT) placement for gastric decompression, heparin drip for VTE and PE and blood transfusion for ongoing anemia in the setting of vaginal bleeding. Transthoracic echocardiography showed a mobile target in the RA and normal biventricular function. The patient developed acute kidney injury with rising creatinine. When her oliguria progressed to anuria on hospital Day 3, she was urgently taken to the operating room for exploratory laparotomy, resection of pelvic mass, total abdominal hysterectomy (TAH), bilateral salpingo-oophorectomy (BSO) and IVC tumor/thrombus resection via sternotomy on cardiopulmonary bypass (CPB).
Hemodynamic goals of anesthetic induction included ensuring adequate preload to the heart in the setting of a large tumor burden causing under filling of the left ventricle (LV) and maintaining adequate heart rate. Hence, 250-ml crystalloid fluid bolus was given prior to induction and a preinduction arterial line was placed to assess continuous blood pressure. Following adequate gastric decompression via NGT, the patient underwent rapid sequence induction and intubation with propofol and succinylcholine. A 12-French dialysis catheter and 8-French double-lumen central line were placed in her right internal jugular vein for vascular access. The former was connected to a rapid volume infuser. Given the possibility of dislodgement of the tumor from the RA into the pulmonary artery during the prebypass period causing saddle embolus and cardiogenic shock, guidewires were placed in both femoral artery and veins for ease of venous and arterial cannulation for possible emergent CPB.
Continuous intraoperative transesophageal echocardiography (TEE) was performed. Postinduction TEE showed a large mobile mass of heterogeneous echogenicity in the RA abutting the tricuspid valve, mild tricuspid insufficiency, occlusive thrombus in the IVC and LV ejection fraction of 40% (Figs 2 and 3; Supplementary Materials S1 and S2). Given the location, size and mobility of the mass in the RA, the decision was made to remove it on CPB to minimize the risk of large embolus and significant blood loss. During the prebypass period, following laparotomy and drainage of abdominal ascites, the patient required significant colloid repletion with 5% albumin and blood products for hypotension, underfilled LV on TEE and low central venous pressure.
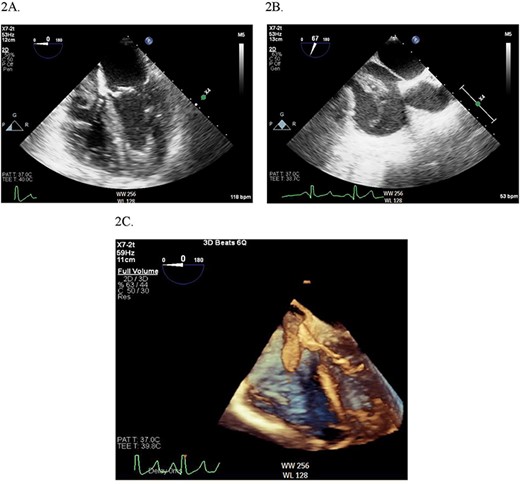
(A) Midesophageal four chamber TEE view showing large mobile mass of heterogeneous echogenicity in the RA. (B) Another view of the RA mass in a midesophageal bicaval view. (C) 3D illustration of the RA mass.
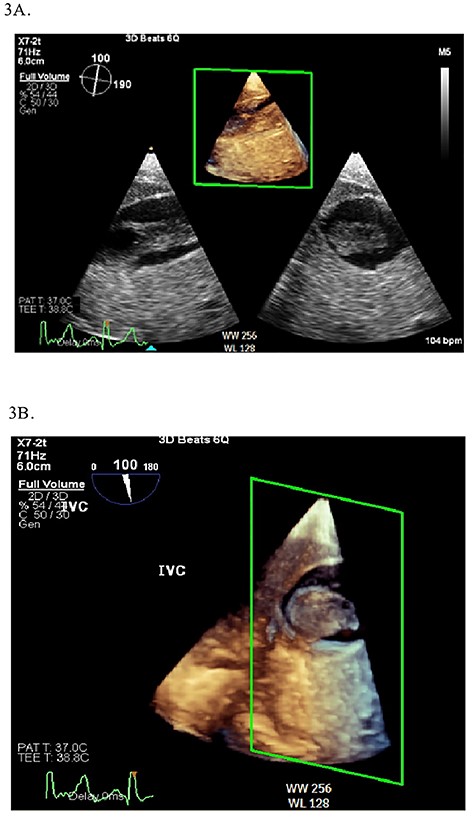
2D (A) and 3D (B) illustration of the IVC thrombus in long and short-axis views.
The tumor appeared necrotic and ruptured into the peritoneal cavity during laparotomy. After tumor mobilization in the abdomen, a TAH–BSO was performed by the gynecology oncology team, and it appeared that the cancer was arising from the right ovary with extension into the gonadal vein and IVC. Mobilization from the iliac bifurcation to the retrohepatic IVC was completed. A median sternotomy was then performed by the cardiothoracic surgeon in preparation for IVC tumor thrombectomy. CPB was established via central aortic cannulation and venous drainage via superior vena cava cannulation and femoral venous cannulation in the IVC below the tumor/thrombus. Following initiation of bypass, a right atriotomy was performed and a large tumor/thrombus extending from the IVC into the RA was identified. A cavotomy was performed in the IVC and the tumor was removed en bloc thorough the RA (Fig. 4). The RA was closed in two layers with monofilament suture. The IVC was reconstructed using an aortic homograft by the vascular surgery team. The patient was weaned off CBP. A portion of the ileum suspicious of tumor extension was resected and primary anastomosis was performed.
The postbypass period was complicated by significant coagulopathy requiring large volume blood transfusion. In total, the patient received 17 units of packed red blood cells, 10 units of fresh frozen plasma, 6 units of platelets, 3 units of cryoprecipitate and 0.5 mg recombinant factor VIIa. Intraoperative viscoelastic testing, platelet count and fibrinogen levels were obtained to assist with administration of these products. The chest and abdomen were closed following correction of coagulopathy. Postbypass TEE showed moderate right ventricular dysfunction requiring inhaled nitric oxide and inotropic support with epinephrine and dobutamine, moderate tricuspid insufficiency and LV function of 50%. She was transferred to the cardiothoracic intensive care unit for postoperative management.
The patient was extubated on postoperative Day 1. Total parental nutrition was initiated for nutritional support as she was profoundly malnourished. Pathology report showed carcinosarcoma of the right ovary with extension to the uterus and tumor/thrombus extending through the gonadal vein into the IVC and RA. There was also involvement of the small bowel and pelvic and aortic lymph nodes. Due to her pathology, she received adjuvant chemotherapy a month after surgery.
DISCUSSION
GCS are rare and aggressive tumors. The majority of patients with ovarian carcinosarcomas are postmenopausal and present with advanced stage intra-abdominal disease [4]. Cytoreductive surgery to remove disease followed by platinum-based chemotherapy appears to improve outcomes, but ovarian carcinosarcoma remains a highly lethal disease with a median survival of about 15 months [2].
The most common gynecological malignancy with tumor/thrombus extension to the heart is intravenous leiomyomatosis [6]. Invasion of renal cell cancer (RCC) into the RA is seen in 1% of affected patients [7]. In contrast to RCC, GCS is a more aggressive and fast-growing tumor. Intracardiac involvement of GCS has only been described once in a patient with progressive dyspnea who was found to have metastases into the left atrium and LV [5]. Molacek et al. [8] reported GCS invading the IVC requiring partial resection of the vena cava. We have reported the second case of GCS extending into the heart and the first case of GCS invading the IVC and contiguously extending into the RA. The use of intraoperative TEE was instrumental in assessing the extent and mobility of the mass and allowed the multidisciplinary team to shape the surgical approach, which in this case included performing the IVC thrombectomy and intracardiac excision while on CPB.
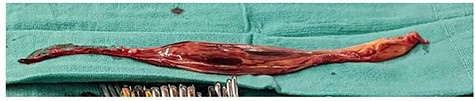
Intraoperative specimen of IVC tumor/thrombus that extends into the right atrium
Intraoperative goals of hemodynamic management included maintenance of adequate intravascular volume in the setting of ongoing blood loss, third spacing and insensible fluid loss, continuous TEE monitoring RA mass during the prebypass period to rule out saddle embolus as a possible cause of unexplained hypotension and assessment of ventricular function during both pre- and postbypass periods for inotropic support need. Coagulopathy can occur during the post-CPB period due to pump run, disseminated intravascular coagulopathy from significant tumor burden and extensive surgery and hypothermia. Intraoperative labs including platelet count, fibrinogen levels and viscoelastic hemostatic assays can assist with deciding which products to administer and can help assess the response of the coagulation cascade to the given products. Given the significant risk of hypothermia in a patient undergoing both laparotomy and sternotomy, it is important to ensure normothermia prior to coming off CPB. The use of underbody forced-air warming blankets, warming blood products that are being administered to the patient, warming the operating room temperature and the use of a heated humidification system for inspiratory gases can assist with ensuring normothermia. Lastly, but importantly, it is essential to discuss the anesthetic concerns with the multidisciplinary surgical teams preoperatively and maintain communication during the procedure.
In conclusion, we present a rare case of ovarian GCS with tumor/thrombus extension into the IVC and RA. The patient’s unique presentation poses significant intraoperative challenges requiring a multidisciplinary team approach to surgical resection, along with anesthetic concerns in maintaining adequate hemodynamics, correcting ongoing volume loss and coagulopathy and assessment of intracardiac tumor burden using TEE.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
References
- hemodynamics
- transesophageal echocardiography
- right atrium
- blood coagulation disorders
- carcinosarcoma
- genital neoplasms, female
- gonads
- intraoperative care
- neoplasm metastasis
- surgical procedures, operative
- inferior vena cava
- neoplasms
- thrombus
- vaginal hemorrhage
- abdominal swelling
- mobility
- en bloc resection



