-
PDF
- Split View
-
Views
-
Cite
Cite
Nicole Shockcor, Rumbidzayi Nzara, Anam Pal, Emanuele Lo Menzo, Mark D Kligman, Operative approach to intestinal malrotation encountered during laparoscopic gastric bypass, Journal of Surgical Case Reports, Volume 2020, Issue 12, December 2020, rjaa466, https://doi.org/10.1093/jscr/rjaa466
Close - Share Icon Share
Abstract
Congenital anomalies of midgut rotation are uncommon with a 0.2–0.5% incidence. Intestinal malrotation (IM) presents a unique challenge in bariatric surgery during laparoscopic gastric bypass (LRYGB), and familiarity with alternatives allows for safe laparoscopic intervention. IM was encountered in 5 of 1183 (0.4%) patients undergoing surgery. Once IM was suspected, a standardized approach was applied: rightward shift of ports, confirmation of IM by the absence of the ligament of Treitz, identification of the duodenojejunal junction, lysis of Ladd’s bands, mirror-image construction of the Roux limb and construction of the gastrojejunal anastomosis. Forty percent were male, age 33 ± 8 years, with body mass index 50 kg/m2 (37–75 kg/m2). IM was identified preoperatively in two patients (40%). All operations were completed laparoscopically. Despite the finding of IM, successful laparoscopic completion of gastric bypass can be anticipated if the surgeon has an understanding of the anatomic alterations and a strategy for intraoperative management.
INTRODUCTION
Anomalies of midgut rotation are uncommon congenital abnormalities with a reported incidence of 0.2–0.5%. Although most symptomatic cases present with evidence of obstruction within the first year of life, 15% of symptomatic cases present in adulthood. The number of individuals remaining asymptomatic throughout life is unknown [1].
The midgut rotation anomalies, when encountered unexpectedly during bariatric operations, can provide the bariatric surgeon unique diagnostic and technical challenges; the surgeon must identify the specific anomaly and then modify the operative approach as appropriate. These challenges are further exacerbated by the physical limitations—specifically those relating to retraction, palpation and visualization—imposed by the laparoscopic approach. However, successful laparoscopic management can be anticipated if the surgeon has an understanding of the anatomic alterations associated with intestinal malrotation (IM) and a strategy for their management.
In this report, we describe five cases of successful laparoscopic Roux-en-Y gastric bypass (LRYGB) in patients with midgut malrotation. We include a review of the literature and provide an algorithm strategy for intraoperative management.
CASE SERIES
The mean age of the five patients included in this case series was 33 ± 8 years (Table 1) and 40% of patients were male. The median body mass index was 50 kg/m2 (range 37–75 kg/m2). Co-morbidities of the patients included gastroesophageal reflux disease, hypercholesterolemia, multiple arthralgias, obstructive sleep apnea and hypertension. Previous abdominal surgery among the patients included a tubal ligation, an open appendectomy and two cesarean sections.
Overview of midgut anomalies encountered perioperatively in cases of laparoscopic gastric bypass
| Author . | Age (years) . | Gender . | BMI (kg/m2) . | Timing of diagnosis . | Type of anomaly . | Surgical technique . |
|---|---|---|---|---|---|---|
| Hamad et al. [5] | 26 | Female | 50.7 | Intraoperative | Malrotation | Laparoscopic |
| Gibbs et al. [3] | 40 | Female | 50 | Intraoperative | Malrotation | Laparoscopic |
| Alam et al | 40 | Female | 50 | Intraoperative | Laparoscopic | |
| Haque et al. [2] | 56 | Female | 44 | Preoperativea | Malrotation | Laparoscopic |
| Haque et al | 32 | Female | 54 | Intraoperative | Malrotation | Laparoscopic |
| Palepu et al. [1] | 49 | Female | 42 | Intraoperative | Malrotation | Conversion to open |
| Palepu et al | 45 | Female | 42 | Intraoperative | Mixed rotation | Laparoscopic |
| Palepu et al | 54 | Female | 41 | Intraoperative | Reverse rotation | Conversion to open |
| Palepu et al | 29 | Female | 62 | Intraoperative | Malrotation | Laparoscopic |
| James et al. [4] | 30 | Female | 44.7 | Intraoperative | Malrotation | Laparoscopic |
| Tayyem et al. [6] | 47 | Female | 36 | Intraoperative | Malrotation | Laparoscopic |
| Gagne et al. [9] | 38 | Female | 48 | Intraoperative | Malrotation | Laparoscopic |
| Sucandy et al. [7] | 42 | Female | 41 | Intraoperative | Malrotation | Conversion to open |
| Kassir et al. [8] | c | c | c | Intraoperative | Malrotation | Laparoscopic |
| Kassir et al | c | c | c | Intraoperative | Malrotation | Laparoscopic |
| Prathanvanich [9] | 59 | Male | 43.4 | Preoperativea | Non rotation | Laparoscopic |
| Patricio et al. [10] | 45 | Female | 40 | Intraoperative | Malrotation | Laparoscopic |
| Patricio et al | 47 | Female | 42 | Intraoperative | Malrotation | Laparoscopic |
| Current Report | 41 | Female | 46 | Preoperativeb | Malrotation | Laparoscopic |
| Current Report | 35 | Female | 43 | Preoperativeb | Malrotation | Laparoscopic |
| Current Report | 41 | Male | 48 | Intraoperative | Malrotation | Laparoscopic |
| Current Report | 26 | Male | 75 | Intraoperative | Malrotation | Laparoscopic |
| Current Report | 24 | female | 42 | Intraoperative | Malrotation | Laparoscopic |
| Author . | Age (years) . | Gender . | BMI (kg/m2) . | Timing of diagnosis . | Type of anomaly . | Surgical technique . |
|---|---|---|---|---|---|---|
| Hamad et al. [5] | 26 | Female | 50.7 | Intraoperative | Malrotation | Laparoscopic |
| Gibbs et al. [3] | 40 | Female | 50 | Intraoperative | Malrotation | Laparoscopic |
| Alam et al | 40 | Female | 50 | Intraoperative | Laparoscopic | |
| Haque et al. [2] | 56 | Female | 44 | Preoperativea | Malrotation | Laparoscopic |
| Haque et al | 32 | Female | 54 | Intraoperative | Malrotation | Laparoscopic |
| Palepu et al. [1] | 49 | Female | 42 | Intraoperative | Malrotation | Conversion to open |
| Palepu et al | 45 | Female | 42 | Intraoperative | Mixed rotation | Laparoscopic |
| Palepu et al | 54 | Female | 41 | Intraoperative | Reverse rotation | Conversion to open |
| Palepu et al | 29 | Female | 62 | Intraoperative | Malrotation | Laparoscopic |
| James et al. [4] | 30 | Female | 44.7 | Intraoperative | Malrotation | Laparoscopic |
| Tayyem et al. [6] | 47 | Female | 36 | Intraoperative | Malrotation | Laparoscopic |
| Gagne et al. [9] | 38 | Female | 48 | Intraoperative | Malrotation | Laparoscopic |
| Sucandy et al. [7] | 42 | Female | 41 | Intraoperative | Malrotation | Conversion to open |
| Kassir et al. [8] | c | c | c | Intraoperative | Malrotation | Laparoscopic |
| Kassir et al | c | c | c | Intraoperative | Malrotation | Laparoscopic |
| Prathanvanich [9] | 59 | Male | 43.4 | Preoperativea | Non rotation | Laparoscopic |
| Patricio et al. [10] | 45 | Female | 40 | Intraoperative | Malrotation | Laparoscopic |
| Patricio et al | 47 | Female | 42 | Intraoperative | Malrotation | Laparoscopic |
| Current Report | 41 | Female | 46 | Preoperativeb | Malrotation | Laparoscopic |
| Current Report | 35 | Female | 43 | Preoperativeb | Malrotation | Laparoscopic |
| Current Report | 41 | Male | 48 | Intraoperative | Malrotation | Laparoscopic |
| Current Report | 26 | Male | 75 | Intraoperative | Malrotation | Laparoscopic |
| Current Report | 24 | female | 42 | Intraoperative | Malrotation | Laparoscopic |
aDiagnosis by history.
bDiagnosis by upper gastrointestinal series.
cNot reported.
Overview of midgut anomalies encountered perioperatively in cases of laparoscopic gastric bypass
| Author . | Age (years) . | Gender . | BMI (kg/m2) . | Timing of diagnosis . | Type of anomaly . | Surgical technique . |
|---|---|---|---|---|---|---|
| Hamad et al. [5] | 26 | Female | 50.7 | Intraoperative | Malrotation | Laparoscopic |
| Gibbs et al. [3] | 40 | Female | 50 | Intraoperative | Malrotation | Laparoscopic |
| Alam et al | 40 | Female | 50 | Intraoperative | Laparoscopic | |
| Haque et al. [2] | 56 | Female | 44 | Preoperativea | Malrotation | Laparoscopic |
| Haque et al | 32 | Female | 54 | Intraoperative | Malrotation | Laparoscopic |
| Palepu et al. [1] | 49 | Female | 42 | Intraoperative | Malrotation | Conversion to open |
| Palepu et al | 45 | Female | 42 | Intraoperative | Mixed rotation | Laparoscopic |
| Palepu et al | 54 | Female | 41 | Intraoperative | Reverse rotation | Conversion to open |
| Palepu et al | 29 | Female | 62 | Intraoperative | Malrotation | Laparoscopic |
| James et al. [4] | 30 | Female | 44.7 | Intraoperative | Malrotation | Laparoscopic |
| Tayyem et al. [6] | 47 | Female | 36 | Intraoperative | Malrotation | Laparoscopic |
| Gagne et al. [9] | 38 | Female | 48 | Intraoperative | Malrotation | Laparoscopic |
| Sucandy et al. [7] | 42 | Female | 41 | Intraoperative | Malrotation | Conversion to open |
| Kassir et al. [8] | c | c | c | Intraoperative | Malrotation | Laparoscopic |
| Kassir et al | c | c | c | Intraoperative | Malrotation | Laparoscopic |
| Prathanvanich [9] | 59 | Male | 43.4 | Preoperativea | Non rotation | Laparoscopic |
| Patricio et al. [10] | 45 | Female | 40 | Intraoperative | Malrotation | Laparoscopic |
| Patricio et al | 47 | Female | 42 | Intraoperative | Malrotation | Laparoscopic |
| Current Report | 41 | Female | 46 | Preoperativeb | Malrotation | Laparoscopic |
| Current Report | 35 | Female | 43 | Preoperativeb | Malrotation | Laparoscopic |
| Current Report | 41 | Male | 48 | Intraoperative | Malrotation | Laparoscopic |
| Current Report | 26 | Male | 75 | Intraoperative | Malrotation | Laparoscopic |
| Current Report | 24 | female | 42 | Intraoperative | Malrotation | Laparoscopic |
| Author . | Age (years) . | Gender . | BMI (kg/m2) . | Timing of diagnosis . | Type of anomaly . | Surgical technique . |
|---|---|---|---|---|---|---|
| Hamad et al. [5] | 26 | Female | 50.7 | Intraoperative | Malrotation | Laparoscopic |
| Gibbs et al. [3] | 40 | Female | 50 | Intraoperative | Malrotation | Laparoscopic |
| Alam et al | 40 | Female | 50 | Intraoperative | Laparoscopic | |
| Haque et al. [2] | 56 | Female | 44 | Preoperativea | Malrotation | Laparoscopic |
| Haque et al | 32 | Female | 54 | Intraoperative | Malrotation | Laparoscopic |
| Palepu et al. [1] | 49 | Female | 42 | Intraoperative | Malrotation | Conversion to open |
| Palepu et al | 45 | Female | 42 | Intraoperative | Mixed rotation | Laparoscopic |
| Palepu et al | 54 | Female | 41 | Intraoperative | Reverse rotation | Conversion to open |
| Palepu et al | 29 | Female | 62 | Intraoperative | Malrotation | Laparoscopic |
| James et al. [4] | 30 | Female | 44.7 | Intraoperative | Malrotation | Laparoscopic |
| Tayyem et al. [6] | 47 | Female | 36 | Intraoperative | Malrotation | Laparoscopic |
| Gagne et al. [9] | 38 | Female | 48 | Intraoperative | Malrotation | Laparoscopic |
| Sucandy et al. [7] | 42 | Female | 41 | Intraoperative | Malrotation | Conversion to open |
| Kassir et al. [8] | c | c | c | Intraoperative | Malrotation | Laparoscopic |
| Kassir et al | c | c | c | Intraoperative | Malrotation | Laparoscopic |
| Prathanvanich [9] | 59 | Male | 43.4 | Preoperativea | Non rotation | Laparoscopic |
| Patricio et al. [10] | 45 | Female | 40 | Intraoperative | Malrotation | Laparoscopic |
| Patricio et al | 47 | Female | 42 | Intraoperative | Malrotation | Laparoscopic |
| Current Report | 41 | Female | 46 | Preoperativeb | Malrotation | Laparoscopic |
| Current Report | 35 | Female | 43 | Preoperativeb | Malrotation | Laparoscopic |
| Current Report | 41 | Male | 48 | Intraoperative | Malrotation | Laparoscopic |
| Current Report | 26 | Male | 75 | Intraoperative | Malrotation | Laparoscopic |
| Current Report | 24 | female | 42 | Intraoperative | Malrotation | Laparoscopic |
aDiagnosis by history.
bDiagnosis by upper gastrointestinal series.
cNot reported.
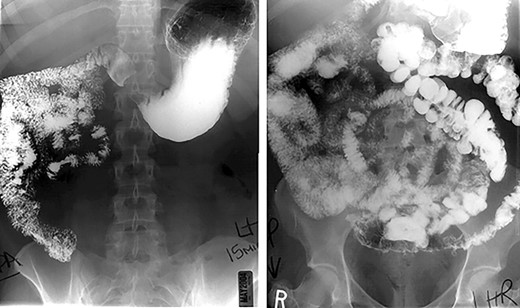
Preoperative work up in two patients (40%) included an upper gastrointestinal series (Fig. 1) revealing midgut non-rotation. The remaining (60%) patients in the series did not endorse gastrointestinal symptoms necessitating an upper gastrointestinal series, IM was identified intraoperatively. In the absence of preoperative diagnosis, malrotation was discovered intraoperatively when the entire greater omentum was found to the left of midline. Confirmation of the diagnosis was based on finding the entire small intestine to the right of midline, the cecum and ascending colon at midline or to the left of midline and the absence of a ligament of Treitz.
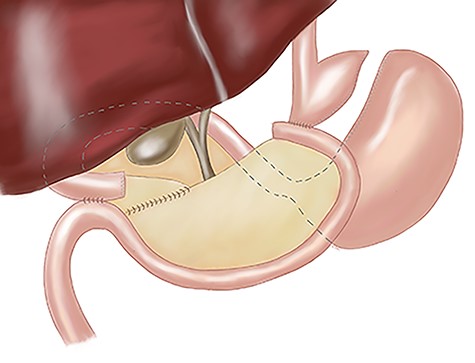
The operative course went as follows for those patients with IM discovered preoperatively: first, primary access to the peritoneal cavity was established with a 12 mm optical trocar placed approximately 4 cm to the left of the midline, 15 cm inferior to the xyphoid process. Second, a 5 mm port was placed in the epigastrium for liver retraction. Third, remaining ports (Fig. 2b) were slightly shifted to the right of our standard positions by 3–4 cm: 5 and 12 mm ports were placed in the right upper quadrant for the surgeon to complete the supramesocolic portion of the procedure. Fourth, 5 and 12 mm ports were placed in the left upper quadrant for the surgeon to complete the inframesocolic portion of the procedure. The first assistant used the ports opposite to the surgeon. Fifth, using these port positions, the surgeon can typically complete the upper abdominal portion of the operation from the patient’s right side and the lower abdominal portion of the operation from the patient’s left side. On the occasion that the orientation of the gastrojejunostomy does not provide adequate access for stapler placement or suturing from the patient’s right side, the surgeon can complete the anastomosis from the patient’s left. Sixth, a 15 ml gastric pouch was completed from the patient’s right side. Inspection of the lower abdomen confirmed the diagnosis of midgut non-rotation. The pylorus was identified and multiple Ladd’s bands were encountered. The entire small intestine was found in the right abdomen. With no rotation, the cecum is typically found in the midline of the lower abdomen and the majority of the remaining colon was often obscured by omentum. Seventh, the jejunojejunostomy was constructed using a ‘mirror image’ approach with the surgeon working from the patient’s left side. Our approach was to add 20 cm to our standard biliopancreatic limb length to account for mobility of the proximal small intestine related to the absence of the ligament of Treitz. Following division of Ladd’s bands, the pylorus is identified, and the jejunum transected 80 cm distal. A 100 cm Roux limb is right-oriented with a right-sided biliopancreatic limb and an antiperistaltic jejunojejunostomy. The anastomosis was constructed using a linear-stapler technique with sutured enterostomy closure; the mesenteric defect was closed with a running non-absorbable suture. The Roux limb was passed in an antegastric, paracolic fashion to allow construction of the gastrojejunostomy. The anastomosis was completed using the same technique as the jejunonejunostomy (Fig. 3). This was completed from the left side of the patient.
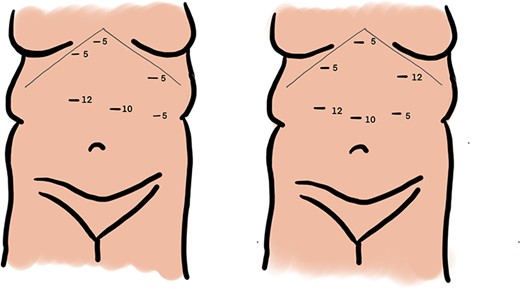
Port placement during laparoscopic Roux-en Y gastric bypass in setting of malrotation anatomical variant, standard port sites (a) and modified port sites (b).
No significant postoperative morbidity or mortality was reported in any of the patients in this series. The mean length of stay was 1.6 ± 0.6 days. Body mass index (BMI) decreased expectantly, with the exception of one patient who was lost to follow up after 3 weeks.
DISCUSSION
The overall incidence of malrotation in the bariatric surgery population has been reported in two series: Palepu et al. [1] reported that midgut malrotation was found in 4 of 504 (0.8%) bariatric surgery patients and, in the current series, midgut malrotation was encountered in 5 of 1183 (0.4%) patients undergoing laparoscopic Roux-en-Y gastric bypass. IM encountered during laparoscopic gastric bypass has been detailed in 23 patients, including the five patients in the current series (see Table 1). Twenty of these patients (87%) had successful laparoscopic completion of their procedures while the remaining three underwent conversion to open gastric bypass. Malrotation was the predominant anomaly, present in 83% of the population. The diagnosis was known preoperatively in only four (17%) patients.
In order to understand the anatomical aberrations associated with IM familiarity with intestinal embryology is essential. The gastrointestinal tract is derived from the endodermal gut tube formed in the fourth week of development. Blood supply to the abdominal portion is provided by vitelline artery derivatives—the celiac, superior mesenteric, and inferior mesenteric arteries. The midgut, supplied by the superior mesenteric artery, differentiates into distal duodenum, jejunum, ileum, ascending colon and the proximal two-thirds of the transverse colon. During the sixth week of development, the future ileum lengthens more rapid than can be accommodated by the more slowly expanding peritoneal cavity creating an anteriorly projecting hairpin loop of intestine—the primary intestinal loop. This differential growth causes herniation of the primary intestinal loop into the umbilicus with an associated 90° counterclockwise rotation—as viewed from anteriorly—around the superior mesenteric artery. The future small intestine now lies on the embryo’s right while the future colon lies on the left. After further differentiation and elongation, the midgut returns to the abdominal cavity while undergoing an additional 180° counterclockwise rotation, thus completing a counterclockwise rotation totaling 270°, during the tenth week. The ascending and descending portions of the colon become retroperitoneal secondarily due to shortening of the dorsal mesentery which eventually fuses to the retroperitoneum [2].
Anomalies of midgut rotation may be categorized into three general patterns—reverse rotation, non-rotation, and mixed rotation. Reverse rotation of the midgut results when the initial 90 degree counterclockwise rotation of the primary intestinal loop is followed by a 180 degree clockwise rotation instead of the normal 180 degree counterclockwise rotation. The net 90 degree clockwise rotation of the midgut reverses the spatial relationship of the duodenum and transverse colon with respect to the superior mesenteric artery. Specifically, the superior mesenteric artery courses anterior to a retroperitonealized transverse colon and posterior to an intraperitoneal distal duodenum.
Non-rotation of the midgut results when the primary intestinal loop while undergoing the initial 90 degree counterclockwise rotation, returns to the peritoneal cavity without further rotation. Failure of the later 180 degree counterclockwise rotation leaves the small intestine on the right side of the body and the entire colon on the left. The cecum is located in the inferior abdomen with the ascending colon being variably retroperitoneal.
Mixed rotation, or malrotation, of the midgut occurs when the cranial limb of the primary intestinal loop undergoes only the initial 90 degree counterclockwise rotation while the caudal limb undergoes only the final 180 degree counterclockwise rotation. The duodenum is localized in the right abdominal cavity and may be covered by a peritoneal band arising from the cecum, which becomes fixed in the midline just inferior to the pylorus [2].
In order to optimally manage these difficult cases, besides a thorough knowledge of the possible anatomic abnormalities, a standardized intraoperative approach is paramount. Our intraoperative approach to midgut non-rotation or malrotation follows a standard pattern with technical considerations related to port placement, identification or confirmation of a midgut rotational anomaly, lysis of Ladd’s bands, construction of the Roux limb, gastrojejunal anastomosis and management of the appendix and gallbladder. If IM is identified preoperatively, our routine gastric bypass ports are shifted rightward, ~3–4 cm to improve operative angles and the left upper quadrant 5 mm port is replaced with a 12-mm port.
The final considerations are whether to perform a simultaneous appendectomy and/or cholecystectomy. Appendectomy has been recommended for pediatric patients with malrotation because the anomalous location of the appendix makes the diagnosis of subsequent appendicitis more difficult. In fact, routine appendectomy is part of the original Ladd procedure. However, no consensus exists with regard to the management of the appendix in the bariatric population with malrotation. Unlike the general patient population, the algorithm for significant abdominal pain after LRYGB includes early imaging studies (specifically computed tomography scan) and, potentially, diagnostic laparoscopy. While unproven, this aggressive algorithm should make delayed diagnosis or misdiagnosis of acute appendicitis less likely [2, 3].
If several authors still advocate incidental appendectomy in malrotation patients, more controversial is incidental cholecystectomy. Routine cholecystectomy during LRYGB has fallen out of favor because of the awkward port placement, the potential complications of cholecystectomy and lack of long-term benefits for the patient. The principal reason for performing routine cholecystectomy in patients with malrotation undergoing LRYGB resides in the close anatomic relationship between the newly constructed Roux limb and the porta hepatis. In fact, as described by other authors, the Roux limb often covers the gallbladder and porta hepatis, not only making the concomitant cholecystectomy more difficult but also increasing the risks of cholecystectomy at a later date. These concerns notwithstanding, routine cholecystectomy and appendectomy during bariatric surgery have a very low yield of pathologic findings and previous reports have shown the lack of economic advantage of incidental appendectomy, especially in patients older than 25 years of age. Consequently, we believe that the addition of appendectomy or cholecystectomy to an already ‘unconventional’ LRYGB cannot be not justified based on the current evidence [3, 4].
Asymptomatic incidentally found midgut rotation abnormalities are rare. When these abnormalities are encountered during LRYGB, successful laparoscopic completion of the procedure can be anticipated if the bariatric surgeon has an understanding of the anatomic alterations and a systematic approach for intraoperative management. The management of the appendix and the gallbladder in these cases is controversial and can potentially add morbidity to the original procedure.



