-
PDF
- Split View
-
Views
-
Cite
Cite
Ali P Mourad, Marie Shella De Robles, Sandra O’Toole, Elizabeth Paver, Robert D Winn, A case of an asymptomatic sacrococcygeal teratoma diagnosed in adulthood, Journal of Surgical Case Reports, Volume 2020, Issue 11, November 2020, rjaa462, https://doi.org/10.1093/jscr/rjaa462
Close - Share Icon Share
Abstract
Sacrococcygeal teratomas are rare congenital tumours that are even more uncommon when present in adulthood. They are derived from residual stem cells in the presacral space that differentiate into clusters of somatic cell. We present the diagnosis, management and post-operative follow-up in a 37-year-old gentleman referred to our department with an incidental finding of a lobulated presacral cystic mass on computed tomography imaging. Magnetic resonance imaging and fluorodeoxyglucose (FDG)-positron emission tomography (PET) scans were performed to further characterize the lesion. The decision was then made for surgical excision and the specimen along with the coccyx was retrieved en-bloc via a trans-sacral surgical approach. Histopathology of the mass uncovered the presence of squamous, respiratory and prostatic epithelium consistent with the diagnosis of a sacrococcygeal teratoma.
INTRODUCTION
Sacrococcygeal teratomas (SCTs) are most commonly present in infantry, and are exceptionally rare in adulthood. By definition, these germ cell tumours originate from pluripotent cells in the presacral space that differentiate into clusters of cells from any of the three primitive cell layers [1]. The estimated incidence is ~1:40 000 with a 4:1 female preponderance. In adults, these tumours are nearly always entirely intrapelvic with no external component [2]. Accordingly, adults usually present when the mass grows to a size sufficient enough to cause compressive symptoms. Here, we present a case where the diagnosis was uncovered incidentally in an adult male presenting to our unit.
CASE REPORT
A 37-year-old gentleman was referred to see a general surgeon with a 4-week history of right groin pain on a background of a previous right inguinal hernia repair. He was otherwise well with no major systemic comorbidities. A computed tomography (CT) scan of the abdomen and pelvis had been organized, which did not demonstrate an abdominal wall defect. Incidentally, there was a 3 cm lobulated cystic mass below the perineal floor on the right side abutting the rectal wall (Fig. 1A). He denied any symptoms of perineal pain or alteration on bowel habit. On examination, his abdomen was benign with no groin cough impulse felt bilaterally. Digital rectal examination (DRE) was normal with no palpable mass.
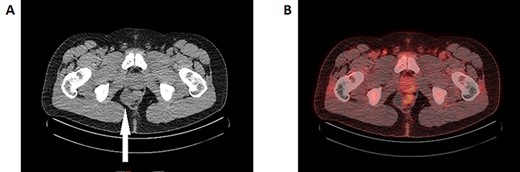
A: Axial slice of the CT pelvis demonstrating a cystic structure in the right presacral space adjacent to the rectum (white arrow). B: FDG-PET/CT reconstruction imaging demonstrating the same cystic lesion adjacent to the FDG-avid rectum. The medial end of the cyst demonstrates FDG-avidity, though it is unclear whether this is intrinsic or artefact from the neighbouring rectum.
A magnetic resonance imaging (MRI) was performed demonstrating a multiseptated perianal cystic collection on the right side ~3 cm from the anal verge extending craniocaudally (Fig. 2A–C). The working diagnosis at the time was a complex congenital cyst. A thin rim of T2-hypointense material was present on its medial wall indicating a solid component, which raises the possibility of malignant potential (Fig. 2B) [3]. A fluorodeoxyglucose (FDG)-positron emission tomography (PET) scan was performed accordingly and while the majority of the cyst was poorly FDG avid, the solid component was difficult to assess due to surrounding artefact from the adjacent rectum (Fig. 1B). The decision was made to proceed with surgical excision.
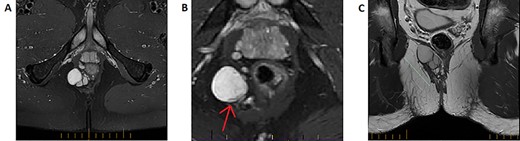
T2-weighted MRI imaging. A: Axial slice taken below the perineal floor demonstrating a multilobulated T2-hyperintense lesion suggestive of a predominantly cystic structure. B: A zoomed slice on the same sequence taken cephalad to (A) indicates that the structure is inhomogeneous—a rim of T2-hypointensity exists at its medial end (red arrow). This represents a solid component and would raise suspicion for malignant potential. C: Coronal slice of the T2 fat-suppression sequence MRI highlighting the cranio-caudal extension of the lobulated structure.
A trans-sacral approach was adopted. The patient was placed in the prone position and a midline incision was made over coccyx and lower sacrum. The muscles were retracted to access the right ischiorectal fossa and the lobulated cyst was dissected off the right sphincter complex to retrieve the specimen. A coccygectomy was subsequently performed. The pelvic floor was repaired with 0-vicryl sutures, the rectum was intact on DRE and the skin was subsequently closed.
Macroscopically, the specimen consisted of a 45 × 35 × 23mm fluctuant cystic mass with serial sectioning indicating it was filled with sebaceous material (Fig. 3A and B). Microscopically, haematoxylin-and-eosin staining was indicative of an SCT containing squamous, transitional and respiratory epithelium (Fig. 3C). No immature or malignant elements were visible. Interestingly, while the coccyx was macroscopically normal, on microscopy there was adjacent fibrous tissue containing prostatic epithelium later confirmed with immunohistochemical staining (Fig. 3D).
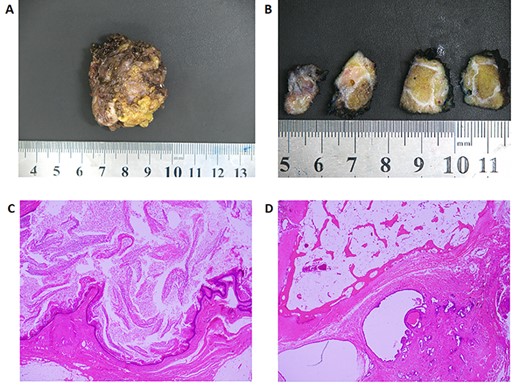
Histology. A: Photo of the macroscopic specimen resected en-bloc. B: Serial sectioning of the specimen shows a cystic structure that is heterogeneous in its composition. C: H&E staining of a section of the cyst containing squamous epithelium and abundant keratin (×20 magnification). D: H&E staining of the coccyx with evidence of prostate glandular tissue adjacent to bone. Immunohistochemistry later showed this tissue to be PSA, PSMA, androgen receptor and NKX3.1 positive confirming the presence of mature prostatic tissue.
The patient’s post-operative recovery was uncomplicated. He was discharged from hospital the following day with a plan for 6-monthly surveillance imaging.
Discussion
SCTs are an uncommon group of congenital tumours that rarely present in the adult male. They are derived from Hensen’s node; a focus of pluripotent stem cells that migrate caudally in the developing embryo and reside anterior to the primitive coccyx [4]. These cells would usually disappear throughout development. Any remaining cells have the potential to evolve into all cell types giving rise to an SCT. Histologically they can be classified as mature, immature and malignant depending on their degree of resemblance to normal tissue, with the vast majority being benign [5]. In adults they clinically manifest with lower back pain, constipation, tenesmus, urinary symptoms or may be asymptomatic as in this case [2, 6].
On suspicion of the diagnosis, the initial imaging modality is a CT and MRI of the pelvis. Together these would help characterize the nature of the lesion (cystic vs solid), its gross anatomical relation, local invasion and the presence of regional lymph node involvement. Differential diagnoses of a presacral mass include an inflammatory abscess, tailgut cyst, metastatic deposit or lesions of neurological origin such as a meningocele [3, 7]. An FDG-PET scan can be used to evaluate for a malignant component, although it may be inconclusive as shown through this case. Serum tumour markers are not usually helpful [7, 8].
Surgical excision is indicated for SCTs that are symptomatic, suspected to be malignant and in women with child-bearing potential [6]. The latter is particularly important due to the potential for obstetric complications during vaginal delivery [9]. Although the final histopathology for our patient was benign, the presence of a solid component on pre-operative imaging has been shown to be associated with malignancy [3]. Thus surgical intervention was warranted. Many however would advocate for operative management for all SCTs as they have an age-dependent risk of malignant transformation [2].
A trans-sacral approach to surgery was adopted for our patient for best accessibility. Transabdominal resection is favoured with larger presacral tumours extending above the mid sacrum [8]. One area of dispute is whether to perform a coccygectomy concurrently. While some advocate to only retrieve the coccyx when it is adjoining the mass [6], others argue that it is the site of origin of the original stem cells and should be removed in order to prevent recurrence [1, 5]. Our case lends weight to the argument for universal coccygeal excision as there was histological evidence of foreign tissue adherent to the coccyx despite appearing macroscopically normal.
The prognosis of benign SCTs following complete resection is excellent, although there is a small lifetime risk of local recurrence. Malignant lesions carry a poorer prognosis and surgery is usually followed by adjuvant therapy tailored to the tissue histology [10]. Resection of our patient’s SCT is thus likely to have been beneficial given our current understanding of this tumour and its natural course. Owing to its rarity, no guidelines exist on the frequency of surveillance imaging post-resection in adulthood. Through this case, we hope to bring to attention the diagnosis and workup of this rare entity in an atypical demographic.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
References
- magnetic resonance imaging
- fluorodeoxyglucose f18
- computed tomography
- stem cells
- epithelium
- adult
- coccyx
- follow-up
- surgical procedures, operative
- diagnosis
- diagnostic imaging
- neoplasms
- prostate
- positron
- sacrococcygeal teratoma
- cystic mass
- somatic cell
- excision
- histopathology tests
- incidental findings



