-
PDF
- Split View
-
Views
-
Cite
Cite
Angela Pallangyo, Jeremia J Pyuza, Alice A Andongolile, Daniel Mbwambo, Jackson P Claver, Ummil-Khairat Koosa, Kennedy Lema, Patrick Amsi, Gilbert Nkya, Alex Mremi, Ovarian malignant mixed Müllerian tumor: a rare case report from Tanzania, Journal of Surgical Case Reports, Volume 2020, Issue 10, October 2020, rjaa406, https://doi.org/10.1093/jscr/rjaa406
Close - Share Icon Share
Abstract
Malignant mixed Müllerian tumor of the ovary is rare aggressive tumor that is histologically defined by the presence of malignant epithelial and stromal components. We report a 37-year-old woman who consulted our facility complaining of abdominal distention and a painful palpable mass over her lower abdomen. Physical examination including computerized tomography revealed a complex cystic mass lesion on the left ovary with extensive omental involvement. Ovarian cancer was suspected and the patient underwent debulking surgery. The histopathology of the specimen revealed a high-grade tumor composed of both malignant epithelial and sarcomatous elements. Both epithelial and stromal components stained positive for p53 immunostaining. Before the initiation of chemotherapy, on 5th day postoperation, the patient was found unresponsive. The stage of the disease seems to be the most important prognostic factor, thus emphasis should be made to identify it in earlier stages.
INTRODUCTION
Malignant mixed Müllerian tumor is uncommon neoplasm of the female genital tract that is histologically defined by the presence of malignant epithelial and stromal elements [1]. Ovarian malignant mixed Müllerian tumors are extremely rare accounting for <1% of all ovarian malignancies [1, 2]. Due to the rarity of this entity, little is known about the disease course and outcome of women diagnosed with this tumor. Because of its aggressive nature accompanied with poor prognosis, few women with this tumor survive longer than few years, thus stage is best predictor [3]. Optimal debulking surgery followed by chemotherapy is the recommended treatment [4, 5].
We report a case of advanced malignant mixed Müllerian ovarian tumor in a 37-year-old female and a glimpse highlight on review of the literature.
CASE REPORT
A 37-year-old woman from rural area Kilimanjaro in northern Tanzania presented to our hospital complaining of abdominal distension for 3 weeks. The distension started gradually and was progressively increasing. It was associated with abdominal pain, early satiety, bloating, loss of appetite and vomiting. However, the patient reported to have normal bowel movements.
On her gynecological history she was gravid 3, para 3, started menarche at 18 years old, although she reported to have had irregular menstrual cycle. During the course of illness she reported to have had per vaginal discharge of pus stained with blood. On past medical history she had history of appendectomy done 9 years ago. Family and social history was unremarkable.
On examination she had normal vital signs except for pulse rates, which were high (127 beats per minute). Per abdomen examination, she had symmetrical distended abdomen, tender at left iliac fossa with positive fluid thrill. Laboratory investigations revealed the hemoglobin level of 14.3 g/dl (12–15 g/dl), white blood count of 13.0 × 7109/l (4–10 × 109/l), platelets of 431 × 109/l (150–400 × 109/l), serum electrolytes, creatinine and urea were within normal ranges, international normalized ratio was 1.77 (0.9–1.2), activated partial thromboplastin time was 34.9 s (20–40 s), cancer antigen (CA-125) was 188.9 U/ml (0–35 U/ml).
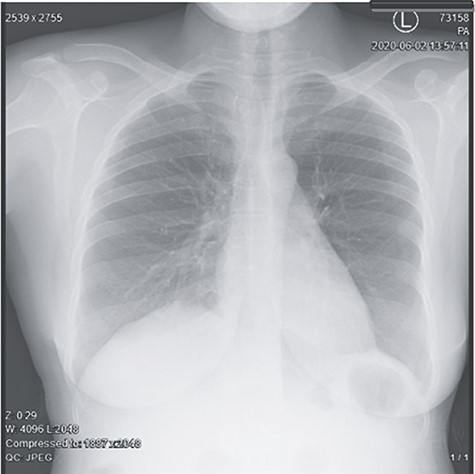
Abdominal pelvic ultrasound revealed ascitic fluid and enlarged uterus. Chest X-ray (Fig. 1) was normal. Computed tomography (Fig. 2) revealed cystic mass arising from left ovary measuring 15.6 × 7.1 × 6.1 cm with multiple soft masses in mesentery, omentum and umbilicus. Diagnosis of left ovarian tumor with peritoneal metastasis was suggested.
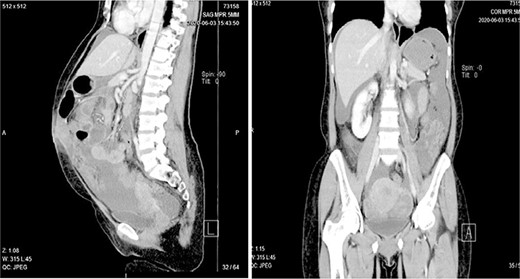
Computed tomography showing a complex cystic mass arising from left ovary with heavy mesentery metastasis.
Explorative laparotomy was done, intra-operatively, and a complex cystic left ovarian mass was found with extensive omental and uterine carcinomatosis seedlings. The liver and spleen were normal. Omentectomy, total abdominal hysterectomy and bilateral salpingo-oophorectomy was done and the samples (Fig. 3) were taken for histopathology. The histopathology results revealed the presence of malignant mixed Müllerian tumor of the ovaries with metastasis to the omentum (Figs 4, 5). The rest of uterus was essentially unremarkable. The tumor cells were strongly positive for p53 immunohistochemistry (on both epithelial and stromal components) (Fig. 6). Furthermore, the epithelial component of the tumor was positive for epithelial membrane antigen immunohistochemistry, whereas stromal element was positive for vimentin.

Hysterectomy specimen with ovarian complex mass and extensive mesentery involvement.
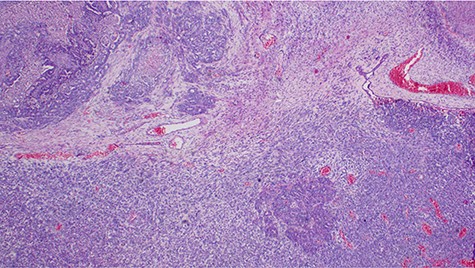
Histopathology of the tumor showing malignant epithelial and sarcomatous elements, hematoxylin and eosin, ×40 magnifications.
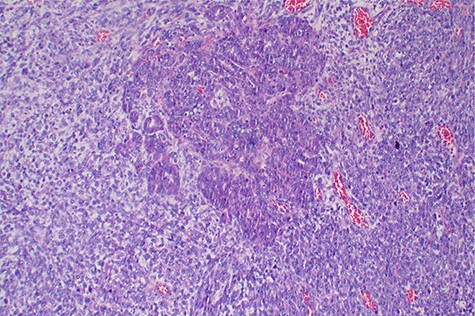
Histopathology of the tumor with malignant epithelial and sarcomatous elements, hematoxylin and eosin, ×100 magnifications.
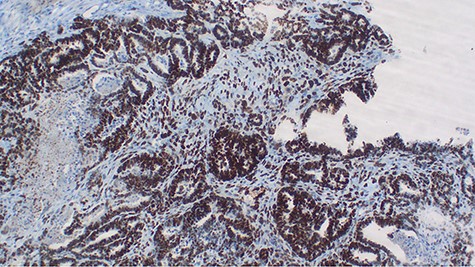
p53 immunohistochemistry highlighting tumor cell positivity on both epithelial and stromal elements, ×40.
After operation, the patient was given 2 units of blood, intravenous (IV) antibiotics and IV fluids. However, the patient succumbed on the 5th day postsurgery where the suspected cause of death being metastatic ovarian cancer.
DISCUSSION
Malignant mixed Müllerian tumor of the ovary is a rare tumor that accounts for ~1% of all ovarian cancers [1, 2]. These tumors are typically large, ranging from 10–20 cm in diameter; our patient’s tumor was 15.6 × 7.1 × 6.1 cm (Fig. 3). The presence of an intimate admixture of malignant epithelial and stromal elements (Figs 4, 5) is the morphological characteristic feature of this tumor. The epithelial element is most commonly a high-grade serous or endometrioid carcinoma, but can be of any of the surface epithelial cell types of ovarian tumors. The stromal component usually contains sheets of hyperchromatic rounded to spindled cells with marked nuclear atypia and a high mitotic index (Fig. 5). Immunohistochemical stains for epithelial markers are often positive in the sarcomatous component, as it has been observed in the index case (Fig. 6), and their behavior and patterns of spread are similar to high-grade serous carcinomas [3–5].
With regard to the histogenesis origin of this tumor, some authors suggested that the collision of carcinoma and sarcoma indicate that these are two independent tumors. The combination theory hypothesizes that both components are derived from single stem cells that undergo divergent differentiation [2]. The conversion theory suggests that a sarcomatous element is obtained from the carcinoma during the evolution of tumors. The least acceptable view is that the mesenchymal element is just a pseudosarcomatous stromal reaction to an invasive carcinoma [2].
The malignant mesenchymal component can be homologous or heterologous. If the sarcomatous part contains element resembling the Müllerian duct system (e.g. fibrosarcoma, leiomyosarcoma and endometrial stromal sarcoma), it is termed homologous. If the tumor has elements not normally found in the ovary (e.g. cartilage, bone, skeletal muscle differentiation, etc.), it is called heterologous. Our tumor was homologous. It has been suggested that heterologous tumors have a worse prognosis. However, some studies have not shown such association [2, 3].
Some authors have reported that there is no well-established consensus regarding the treatment regimen [6]. In the older literature, these tumors were considered aggressive, rapidly fatal with a median survival of about 1 year, but more recent data suggested that with aggressive debulking surgery together with combination of cisplatin and either a taxane or ifosfamide chemotherapy, survival rates could be improved, approaching those for serous carcinoma [7–9]. The role of therapeutic radiotherapy is not established [9]. When stratified by stage, however, limited data suggest that the prognosis is worse than that for serous carcinoma [8]. Despite the treatment that includes radical surgery and chemotherapy, women with these tumors have a significantly increased risk of death compared with women with epithelial ovarian cancer and very poor prognosis [9, 10].
Contrary to the most reports in literature, which indicated that the tumor is commonly seen in postmenopausal women with a peak in the sixth decade and low parity [4, 6], our patient was within the childbearing age; however, in line to the previous reports, our patient presented with an advanced metastatic disease characterized by aggressive and succumbed shortly after surgery.
CONCLUSION
Malignant mixed Müllerian tumor of the ovary is a rare tumor usually diagnosed at an advanced stage, and survival after diagnosis varies by stage of the disease. Despite aggressive treatment that includes surgery and chemotherapy, women with these tumors have very poor prognosis, emphasizing the need for earlier diagnosis to improve patients ‘survival’.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
A written informed consent to undergo treatment including surgical and chemotherapy as well as to use de-identified patient’s data for academic purposes including publication were provided by the patient’s family. A copy is available for review if requested by editorial office.
CONSENT FOR PUBLICATION
The consent for publication was obtained along with an informed consent from the patient’s family.
AVAILABILITY OF DATA AND MATERIALS
All the information included in the current study is available from the corresponding author upon editorial office request.
ACKNOWLEDGMENT
The authors acknowledge Dr Bariki Mchome and his associates who performed the surgery as well as the patient and her family for allowing us to use the patient’s information in this publication.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.



