-
PDF
- Split View
-
Views
-
Cite
Cite
Kentaro Minegishi, Hiroyoshi Tsubochi, Kohei Hamamoto, Shunsuke Endo, Massive lymphatic leakage after lung cancer surgery via median sternotomy, Journal of Surgical Case Reports, Volume 2019, Issue 7, July 2019, rjz178, https://doi.org/10.1093/jscr/rjz178
Close - Share Icon Share
Abstract
We report a case of intractable chylothorax after right upper lobectomy and nodal dissection via median sternotomy for lung cancer in a 67-year-old man. Lymphangiography (LAG) with lipiodol and sequential computed tomography showed the thoracic duct in the left posterior mediastinum and massive lymphatic leakage in the anterior and middle mediastinum. The Chylous leakage was resolved by LAG with lipiodol. Our findings suggest that variation of the thoracic duct should be evaluated by LAG when intractable chylothorax or chylomediastinum develops after anterior mediastinal surgery.
INTRODUCTION
Postoperative chylothorax is caused by direct injury to the thoracic duct or its branches during surgery, especially in the posterior mediastinum near the thoracic duct, as might occur during mediastinal dissection or esophagectomy. Surgical interventions in the anterior mediastinum, such as thymectomy or cardiac surgery, can cause chylothorax and chylomediastinum. To our knowledge, no previous radiological study has clearly visualized postoperative lymphatic leakage in the anterior mediastinum. Here, we describe a case of intractable massive chylothorax after right upper lobectomy and mediastinal dissection via median sternotomy in a patient with lung cancer. In this case, lymphangiography (LAG) and sequential computed tomography (CT) showed massive lymphatic leakage in the anterior and middle mediastinum, which was most likely caused by injury to a well-developed lymphatic network.
CASE REPORT
A 67-year-old man was referred to our hospital after transbronchial biopsy confirmed a diagnosis of non-small cell lung cancer. His physical examination findings and laboratory data were unremarkable. CT revealed a right upper lobe mass (diameter, 4.1 cm) in the apical portion; and thus, invasion of the right subclavian artery was suspected. On clinical staging, the tumor was T4N0M0, Stage IIIA. He underwent right upper lobectomy and mediastinal lymph node dissection via median sternotomy. Complete resection was achieved without concomitant resection of the right subclavian artery. The histological diagnosis was squamous cell carcinoma (pT3aN0M0, Stage IIB). Two drainage tubes were placed in the right thoracic cavity, and a total milky fluid volume of 2000 ml was drained on postoperative day (POD) 2. The volume of chylous drainage did not reduce with total parenteral nutrition. On POD 4, right-sided video-assisted thoracic surgery was performed 1 hour after intake of ice cream, to identify the site of chylous leakage. On intraoperative observation, multiple leakage sites were noted between the trachea and superior vena cava, and these sites were clipped and sealed with fibrin glue. However, the thoracic duct could not be identified in the right posterior mediastinum. After this surgical procedure, the volume of chylous drainage did not decrease. In addition, on POD 5 the patient complained of dyspnea. A chest X-ray revealed left pleural effusion, and a drainage tube was inserted into the left pleural cavity. LAG was performed to identify the leakage site, and the total amount of drained effusion from both sides was 3200 ml/day on POD 6 (2 days after the reoperation). The right inguinal lymph node was punctured with a 23-gauge needle under ultrasound guidance, and a total lipiodol volume of 7 ml was slowly injected under fluoroscopic guidance. CT after LAG revealed that the thoracic duct was located on the left side (Fig. 1). However, a day after LAG, the leakage site could not be clearly identified. Additionally, CT showed accumulation of lipiodol at the anterior mediastinum, which suggested the presence of chylous leakage from the thoracic duct tributaries in the anterior mediastinum (Fig. 2). Bilateral pleural effusion gradually decreased after LAG, and a low-fat diet was started on POD 20. The right and left chest drainage tubes were removed on POD 21 and POD 28, respectively. The patient was eventually discharged 30 days after the first surgery (23 days after LAG). CT performed 2 months after LAG revealed lipiodol retention in the thoracic duct and mediastinum. The patient remains asymptomatic without pleural effusion at 6 months after discharge.
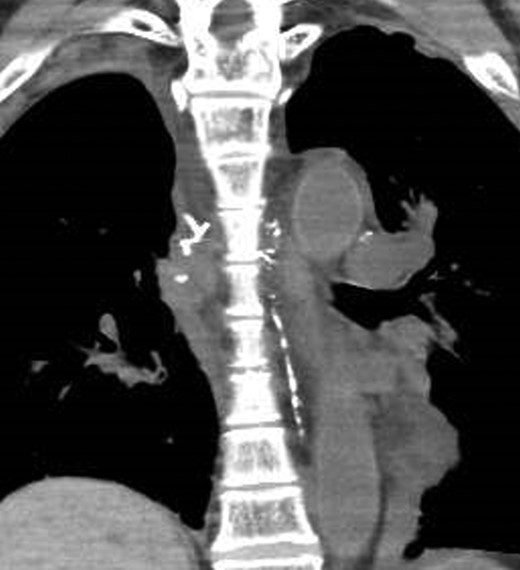
A CT image immediately after lymphangiography shows the thoracic duct coursing in the left thorax (arrow).
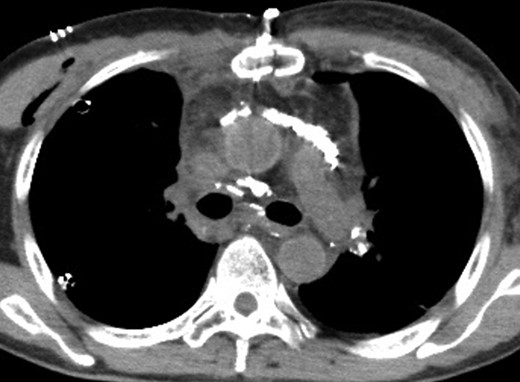
A CT image 1 day after lymphangiography shows lipiodol accumulation in the anterior mediastinum and between the bilateral main bronchi and aorta.
DISCUSSION
Chylothorax has been reported after various thoracic surgeries, and the incidence rate after lung resection with lymph node dissection has been reported to be 1.4–2.3% [1, 2]. The incidence of chylothorax is relatively high after coronary artery bypass grafting that requires harvesting of the left internal thoracic artery, as its proximal segment runs near the thoracic duct [4]. Postoperative chylothorax or chylomediastinum sometimes develops after other anterior mediastinal surgeries, such as thymectomy or other cardiac surgeries that require median sternotomy [3, 4]. However, no previous radiological study has clearly identified the site of chylous leakage after such surgeries. Anatomical variations of the thoracic duct are present in about 10% of patients [5]. In this case, LAG and sequential CT showed the thoracic duct ascending in the left posterior mediastinum and a well-developed lymphatic network from anterior and middle mediastinal structures draining into the thoracic duct immediately before or at its junction with the vein. Thoracic duct variation might be associated with proliferation of the mediastinal lymphatic network. Thus, to avoid repeated surgeries for ligation of the thoracic duct via the right thoracic cavity, thoracic duct variation should be evaluated using LAG.
Massive chylous leakage can lead to dehydration, malnutrition, and sepsis; thus, treatment delay can be potentially life-threatening. Surgery is usually considered when conservative therapy is unsuccessful. A recent report mentioned that patients with postoperative chylous chest tube drainage of 1100 ml or more in 24 hours should be considered for surgical ligation of the thoracic duct. In addition, thoracic duct embolisation might be considered when surgery fails [6].
Conventional LAG is usually used as a diagnostic tool for lymphatic vessel leakage, and several studies have described the therapeutic efficacy of LAG for various types of chyle leakage, including chylothorax [7, 8]. The mechanism by which LAG reduces chyle leakage is not well understood. One hypothesis is that lipiodol retention around the leakage point outside the lymphatic vessel triggers an inflammatory reaction and causes obstruction of the lymphatic vessel. Lipiodol accumulation distal to the point of leakage might act as an embolic agent. Indeed, lipiodol retention in the thoracic duct was observed in our patient even at 2 months after LAG.
In conclusion, we reported a patient with intractable chylothorax after lung cancer surgery via an anterior mediastinal approach, LAG and sequential CT revealed an anatomical variation of the thoracic duct and a rich lymphatic network in the anterior and middle mediastinum. Our findings suggest that variations of the thoracic duct should be evaluated by LAG when intractable chylothorax or chylomediastinum develops after anterior mediastinal surgery.
CONFLICT OF INTEREST STATEMENT
None declared.



