-
PDF
- Split View
-
Views
-
Cite
Cite
Sanjiv Gray, Michael Katzen, Vignesh Vudatha, A case of recurrent pneumatosis intestinalis, Journal of Surgical Case Reports, Volume 2018, Issue 4, April 2018, rjy077, https://doi.org/10.1093/jscr/rjy077
Close - Share Icon Share
Abstract
Pneumatosis intestinalis (PI), defined as free gas in the bowel wall, is associated with autoimmune conditions, drugs, pulmonary disease and many other etiologies. Patients with findings of PI may have variable clinical presentations, ranging from asymptomatic to acute abdomen necessitating urgent surgery. Here, we present the case of an individual with recurrent PI whose suspected etiologies ultimately varied from benign to lethal between visits. We discuss the clinical management of each case, perform post-hoc application of a proposed treatment algorithm, and highlight areas for future research.
INTRODUCTION
Pneumatosis intestinalis (PI) is rare radiological finding that is characterized by free gas in the bowel wall, which could include the esophagus, stomach, small intestine or colon [1]. There are currently two theories that explain this phenomena. One states that gas from the intestinal lumen or lungs dissects the bowel wall via the mediastinum in cases of intestinal obstruction. The second theory postulates that gas-forming bacilli migrate into abdominal wall tears and produce gas within the submucosa [2].
In this report, we discuss case of individual who presents with recurrent PI in the context of two differing clinical presentations.
CASE REPORT
A 64-year-old female presented to the emergency department with a chief complaint of epigastric pain and nausea lasting 24 h. Relevant medical history includes congestive heart failure, interstitial lung disease, chronic obstructive pulmonary disease and prior open cholecystectomy. Home medications included a course of methylprednisolone for acute exacerbation of lung disease. On exam, her abdomen was tender and distended. Labs showed leukocytosis with normal lactate level. Figures 1 and 2 show abdominal computed tomography (CT) without contrast, significant for small bowel obstruction with intramural small bowel pneumatosis and extensive portal venous gas (PVG) within the left hepatic lobe. Emergent laparotomy was performed for acute abdomen. Exploration of the abdominal viscera revealed no evidence of inflammation, necrosis or perforation. The patient continued to have pain after surgery. Upper endoscopy was performed, revealing a non-perforated gastric ulcer which was treated with medical management until the pain resolved.
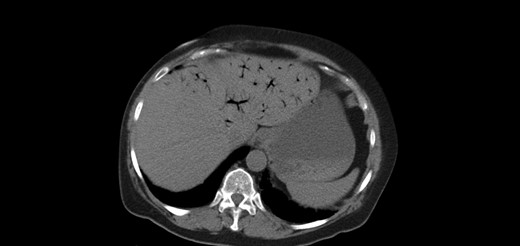
Extensive portal venous gas noted in the left lobe of the liver during the first admission. Laparotomy was negative for bowel ischemia.
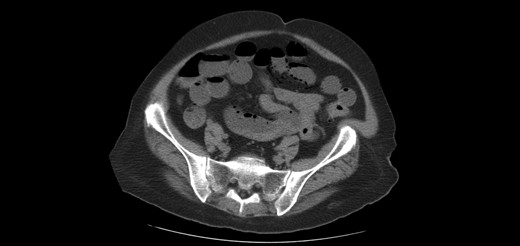
One year later, the patient was brought to the emergency department by her family for altered mental status. They reported 2 days of abnormal behavior and confusion culminating in an episode of bowel incontinence. The patient had difficulty following commands and was complaining of abdominal pain. Initial workup showed tachycardia, hypotension, leukocytosis and elevated creatinine which was treated per sepsis protocol. Her toxicology screen was positive for cocaine. Serum lactate was also elevated. CT brain without contrast showed no acute intracranial process. CT without contrast of the abdomen and pelvis, shown in Figs 3 and 4 , revealed dilated small bowel with gas in the bowel wall. Emergent damage control surgery was performed. There were no signs of gastrointestinal perforation but two segments of necrotic small bowel, 5 feet in total, were removed and the abdomen was sealed with Abthera. Two days later, her abdomen was re-explored and another 40 cm of non-viable small bowel was removed, leaving her with two small bowel anastomosis. Her abdomen was re-explored and closed after another 2 days. She was successfully weaned off the ventilator and continued to improve with supportive care. Final pathology examination revealed extensive necrosis with acute inflammation impacting the serosa at points in the small bowel.
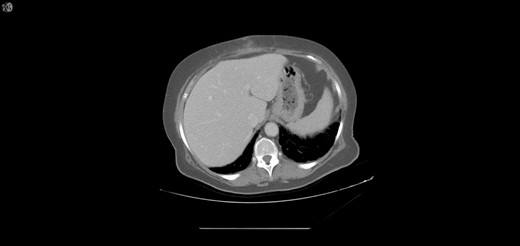
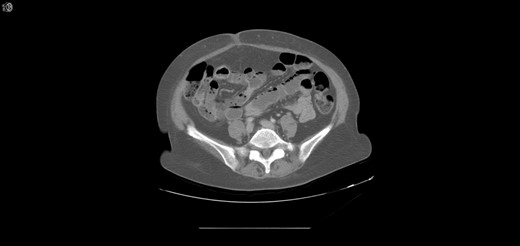
Extensive pneumatosis of the small bowel during the second admission. Approximately 5 feet of ischemic bowel resected.
DISCUSSION
PI is associated with autoimmune conditions, inflammatory bowel diseases, thoracic organ transplants, medications and pulmonary diseases. Roughly 15% of cases are classified as idiopathic. Most patients are asymptomatic but some can present with life-threatening intra-abdominal conditions. PI in small intestines tends to present with vomiting, abdominal distention and weight loss, whereas PI in the colon presents with diarrhea, hematochezia and abdominal discomfort.
Management guidelines for PI are not well established. CT offers greater diagnostic sensitivity over ultrasound or plain film as it can better distinguish between intramural and intraluminal air [3]. Goyal et al. [4] reviewed worrisome features on CT scan and found small bowel location, bowel dilatation, stranding, enhancement, PVG, mesenteric venous gas and moderate mesenteric edema to have statistical significance. Treatment involves deciding whether patient needs surgical intervention or not. Typically patients with clinical signs of peritonitis and metabolic acidosis are candidates for exploratory laparotomy [5]. Lactate levels >2 mmol/L predicts a higher mortality rate in patients with PI [6]. Other ominous clinical features include underlying bowel disease and mechanical cause of the obstruction [4]. Patients without these features may be treated conservatively with antibiotics, elemental diet and oxygen therapy. Asymptomatic patients are not treated as PI usually resolves over time. In all patients, regardless of clinical presentation, the primary cause of PI should be addressed [5].
The patient in this case had risk factors for developing PI: interstitial lung disease, history of smoking and cocaine use. She was also taking corticosteroids, the most common cause of medication-induced PI [7]. Surgical intervention was warranted on the first visit due to acute abdominal signs (i.e. tenderness, distention) with leukocytosis and on second admission due to abdominal pain, hypotension, tachycardia and elevated lactate.
There are no established guidelines or scoring systems that directs management of PI. Wayne et al. reviewed 74 cases of patients with PI or PVG and formulated a decision algorithm based on patient stability, PI or PVG etiology, and vascular risk factors. Once patients are stable and mechanical or gastrointestinal etiologies are ruled out, a vascular risk score is calculated to categorize the PI or PVG as suspected mesenteric ischemia (score > 6), possible mesenteric ischemia (score 4–6) or benign PI (score < 4). The score assigns points to cardiovascular history, abdominal pain, lactate > 3.0 mg/dL, and small bowel pneumatosis on imaging. In this case, the patient had scores of 5 and 8 on the first and second admissions respectively [8]. Based on this algorithm, the patient was in the moderate risk category before receiving a non-therapeutic laparotomy and at a high risk before the second, therapeutic operation. This indicates that the extent of PVG may not be strongly associated with underlying abdominal pathology. There may, however, be a role in using PI and PVG in conjunction with other diagnostic markers to determine which patients require aggressive intervention.
This clinical picture highlights several areas where more research is needed. The multifactorial etiology for PI is poorly understood. There are no systematic reviews at this time which identify risk factors for recurrence of PI. Current treatment algorithms are not clear on the role of PI or PVG in guiding clinical interventions, especially for patients with recurrent episodes.
Acknowledgements
Not applicable.
Conflict of Interest statement
None declared.



