-
PDF
- Split View
-
Views
-
Cite
Cite
Emily F Kelly, Alan A Stein, Xiaoling Charlene Ma, Jose Yeguez, A rare case of perianal granular cell tumor: case report and literature review, Journal of Surgical Case Reports, Volume 2017, Issue 6, June 2017, rjw186, https://doi.org/10.1093/jscr/rjw186
Close - Share Icon Share
Abstract
Granular cell tumor (GCT) is a rare submucosal neoplasm most commonly localized in the oral mucosa; with one-third of all cases found in the tongue, with less than 30 cases of perianal GCT reported in the literature, making it a rare anal neoplasm. Wide local excision is the gold standard of treatment and follow-up includes annual colonoscopy due to the high incidence of reoccurrence. Here we describe a rare case of benign perianal GCT in a 29-year-old female who presented asymptomatically; however, pathology report revealed a S100 positive immunostaining pattern. GCT is an important differential to be included when evaluating a patient with an asymptomatic perianal submucosal lesion. Since GCT and Squamous Cell Carcinoma present with similar pseudoepitheliomatous hyperplasia of the epithelium it is important that a biopsy and immunohistochemical analysis be performed to allow for accurate diagnosis and appropriate treatment.
INTRODUCTION
Granular cell tumor (GCT) is a rare submucosa tumor most commonly found in the oral mucosa; localized to the tongue in one-third of cases [1, 2]. Other sites include the skin, gastrointestinal tract, breast, biliary tract, respiratory tract, genital tract, orbit and mastoid. About 8% of GCT lesions are localized to the gastrointestinal tract and the esophagus accounts for the majority of these cases followed by the large intestine [3, 4]. There are less than 30 cases of perianal GCT reported in the literature, as of 2015, making it a rare anal neoplasm [2, 3, 5]. Most of the reported cases are single case reports; however, the US Armed Forces Institute of Pathology, Johnston et al., reported 75 cases of gastrointestinal GCT, of which 16 cases were localized to the perianal region [2]. GCT is classified as rare submucosa tumor with over 20 proposed histogenetic theories [3]. In 1926, Abrikossoff reported the first five cases of GCT with three of five being localized to the tongue. Abrikossoff postulated that the tumor arose from an embryonic muscle cell, specifically degenerating striated muscle, and identified the tumor as a myoblastic myoma [1, 6]. In 1939, Leroux and Delarue postulated that GCT was not myogenic in origin, but a non-neoplastic accumulation of granular histocytes. Then, in 1935, Feyrter et al. proposed the most current and accepted pathogenesis that the tumor as neural in origin dubbing GCT as a myoblastoma [1]. Gullino et al., in 1949, using immunohistochemistry and electron microscopy, described the myoblastoma as Schwann cell in origin due to its S100, neuron-specific enolase (NSE) and myelin-associated glycoprotein positive staining pattern [1, 4, 5].
Most commonly GCT lesions are confined to the submucosa and present as a benign, asymptomatic, small, non-ulcerated, polypoid, firm lesions most commonly found incidentally on endoscopy/colonoscopy evaluation for hemorrhoids and fissures [2, 4, 5, 7, 8]. About 1–2% of cases are malignant. Although the majority of cases are asymptomatic, Johnston et al. reported that perianal GCT is more likely to present with perianal discomfort and hematochezia than other sites [3, 9]. Females have a 1.5:1 greater prevalence than males and presents between the fourth and sixth decade of life with an equal prevalence among all races [2, 4, 6, 10]. Yamada et al. found that the most important predicting factor for malignancy is size, followed by atypical histology [4, 6, 8]. Benign lesions are often less than 3 cm and have uniform nuclei with the absence of mitotic figures, whereas, a lesions greater than 4 cm were found in 60% of malignant cases [4, 8]. Histological findings in benign and malignant lesions are identical; however, malignant cells can rarely demonstrate cellular necrosis, pleomorphism, enlarged nucleoli, cell elongation, increased nuclear-to-cytoplasmic ratio and mitotic activity [3, 6, 9]. Fanburg-Smith et al. postulated the presence of three or more of these factors that indicate likely metastasis [4]. Metastases are more common with reocurrence of a previously benign lesion spreading via lymphatic or hematogenous dissemination to the lung, liver, bone and lymph nodes [6, 9].
CASE REPORT
A 29-year-old female presented with an enlarging perianal mass over several years. She denied any tenderness, pain or bleeding. Physical examination revealed mobile, elevated, indurated left lateral mass, not attached to the deep planes measuring 3 cm in length in close proximity to the anal verge. At this time the decision to undergo excision and biopsy was made. Under general anesthesia the perianal region was prepped and draped in the usual sterile fashion. The anal mass was identified. The skin around the mass was injected with Marcaine 25% local anesthetic. An ellipitical incision was made around the mass approximately 0.5 cm from the mass edge. The subcutaneous tissue was taken down with electrocautery and the mass was completely removed without complications and sent for pathology. The skin was closed using interrupted subcuticular stiches and Monocryl. Pathology revealed a S100 positive immunostaining pattern with positive and negative controls leading to a diagnosis of GCT (Fig. 1a and b).
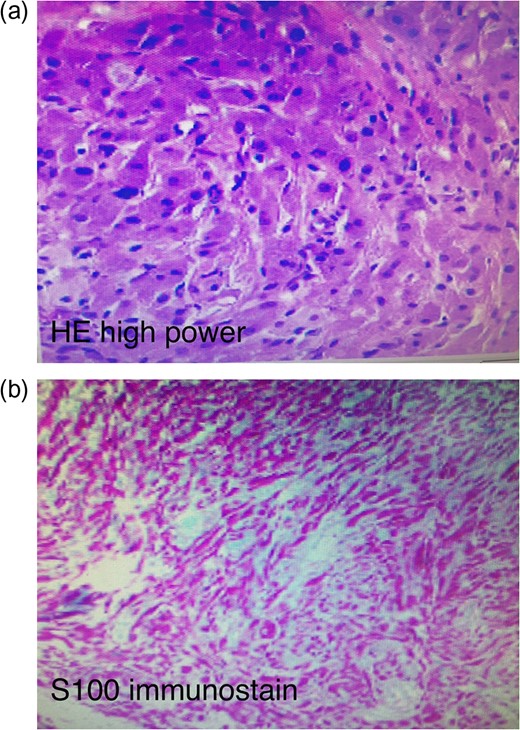
(a) Haemotoxylin and eosin stain of the pathological specimen. (b) S100+ immunostain of the pathological specimen.
DISCUSSION
Early in its course, GCT is commonly misdiagnosed as a quiescent abscess [2, 5]. Squamous cell carcinoma (SCC) must be ruled out due to the pseudoepitheliomatous hyperplasia and overlying acanthosis that GCT demonstrates. GCT can be differentiated from SCC by its keratin, desmin and muscle specific actin negative staining pattern [2, 3, 7, 10]. Diagnosis must be made by biopsy and histological and immunohistochemical analysis [6]. Characteristic features include non-encapsulated large polyhedral/plump/histiocyte-like cells with abundant granular eosinophilic cytoplasm, uniform nucleoli and separated by collagen fibers. The eosinophilic granular cytoplasm is acidophilic, periodic acid–Schiff (PAS) positive and diastase-resistant [10]. Ultrastructural analysis demonstrates that the cytoplasmatic granules are an accumulation of lysosomes similar to those found in Schwann cells [2, 5, 8, 9]. Immunohistochemical diagnosis is made by the S100, NSE, Vimentin and lysosomal marker CD68 positivity that GCT demonstrates in its nucleus and cytoplasm, in line with GCT’s proposed Schwann cell origin [4, 5, 7]. Wan et al. proposed that malignancy can be determined by a p53 greater than 50% and a Ki67 index greater than 10% [3].
Wide local excision is the gold standard treatment of perianal GCT [2–4, 10]. Excision of affected lymph nodes is recommended in patients with lymphatic involvement [9]. Aksoy et al. revealed in nine patients that adjuvant chemotherapy and/or radiation did not alter the disease survival or overall survival in patients with metastasis or recurrence. Furthermore, in colonic GCT it has been proposed that lesions less than 1 cm can be monitored and lesions greater than 1 cm can be treated by endoscopic excision [4, 8]. The prognosis of malignant GCT is poor with a 30–50% mortality rate. Fanburg-Smith et al. reported that the 2-year reoccurrence rate of GCT is 32% for malignant disease and 2–8% for benign disease. This rate increases to 20% in benign lesions with positive margins [6]. Therefore, follow-up with annual colonoscopy and endoscopy is necessary [10].
Perianal GCT is a rare lesion presenting clinicians which is difficult diagnosis. Therefore, GCT is an important differential to be included when evaluating a patient with an asymptomatic perianal submucosal lesion. Since GCT and SCC present with similar pseudoepitheliomatous hyperplasia of the epithelium it is important that a biopsy and immunohistochemical analysis be performed to allow for accurate diagnosis and appropriate treatment. Patients must be closely monitored post-treatment due to the high rate of recurrence.
CONFLICT OF INTEREST STATEMENT
None declared.



