-
PDF
- Split View
-
Views
-
Cite
Cite
Xuan Luu, Samantha Leonard, Kathie-Ann Joseph, Dermatomyositis presenting as a paraneoplastic syndrome with resolution of symptoms following surgical management of underlying breast malignancy, Journal of Surgical Case Reports, Volume 2015, Issue 7, July 2015, rjv075, https://doi.org/10.1093/jscr/rjv075
Close - Share Icon Share
Abstract
Breast cancer is the most common cancer in women in the USA, with the lifetime incidence of 1 in 8 women. Dermatomyositis (DM) is an uncommon idiopathic inflammatory myopathy that can manifest as a paraneoplastic syndrome of an underlying malignancy. Here, we report a case of a patient who presented with breast cancer and DM symptoms. The patient's rash and muscle weakness progressed during the workup of her breast cancer, while she was already started on medical treatment of these symptoms with oral prednisone. Her cutaneous and musculoskeletal improved dramatically following the treatment of her breast cancer. Our case report describes the rapid progression and regression of her symptoms emphasizing the benefit of early diagnosis and treatment of DM as well as the underlying breast cancer.
INTRODUCTION
Dermatomyositis (DM) is an uncommon idiopathic inflammatory myopathy that primarily affects skeletal muscle and skin with well-characterized cutaneous findings. The estimated incidence of DM is ∼1/100 000 [1, 2]. It has been well documented that DM carries an increased risk of malignancy and can present as a paraneoplastic syndrome to multiple types of underlying malignancies [3, 4]. The majority of cases of DM is idiopathic. However, ∼15–30% of cases of DM are manifestations of paraneoplastic syndromes of an underlying malignancy. Here, we present a case of concurrent DM and breast cancer to highlight a rare presentation, progression and regression of symptoms, as well as key components of diagnosis and treatment.
CASE REPORT
The patient is a 47-year-old Polish premenopausal female who presented with complaints of a non-tender palpable left breast mass for 4 months at an outside institution. She underwent a diagnostic mammogram and ultrasound that reported a 2-cm irregular, hypoechoic mass at 12 o'clock. The mass had indistinct borders on ultrasound (Figs. 1,2). Bilateral breast MRI revealed no disease in either axilla or the right breast. There was a 2.2-cm enhancing mass in the left breast at 12:00 that was consistent with the patient's biopsy-proven malignancy (Fig. 3). Upon examination in the Breast Surgery clinic, she underwent a core needle biopsy, which revealed a poorly differentiated triple-negative invasive ductal carcinoma (Fig. 4).
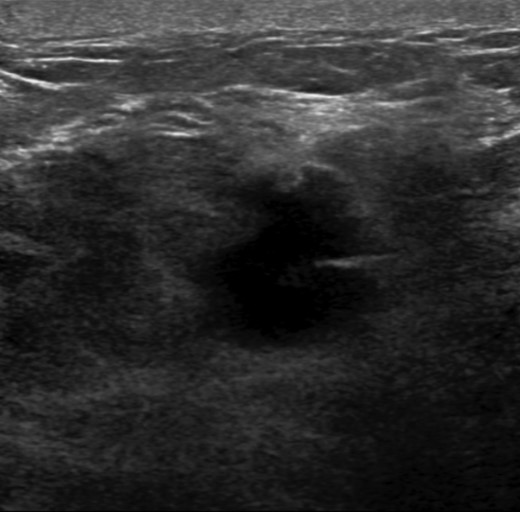
Left breast ultrasound. There is a 1.9 × 1.4 cm irregular mass in the left breast at 11–12:00 6 cm from the nipple.
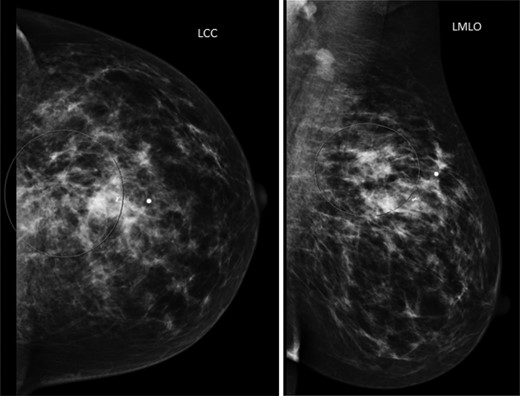
Left mammogram. The circled area revealed a 2-cm irregular mass with biopsy clip noted.
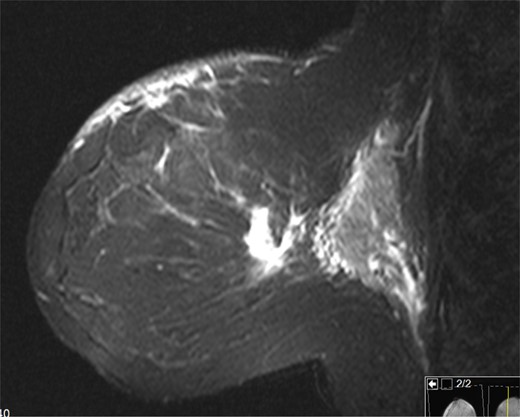
Left breast on MRI. There was a 2.2 cm enhancing mass in the left breast at 12:00 that was consistent with the patient's biopsy-proven malignancy.
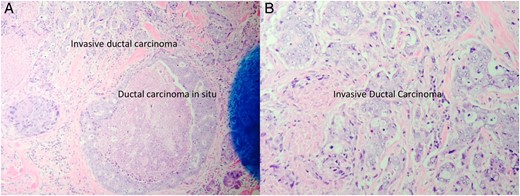
Low power view of the left breast core biopsy (A). High power view of the left breast core biopsy (B).
Two weeks after the biopsy, she complained of new onset pruritic, erythematous rash on her anterior chest, face and back of the hands (Fig. 5). She also reported slowly worsening periorbital edema (Fig. 5A). She was complaining of diffuse myalgias, worse during menstruation. Owing to her worsening symptoms which included cuticular hypertrophy of the hands with associated erythema over the metacarpophalangeal and proximal interphalangeal joints bilaterally (Fig. 5C), she was transferred to the Bellevue Hospital Breast Clinic and referred to the rheumatology clinic for management of her symptoms. Initial laboratories revealed moderately elevated laboratory values (Table 1). At this time, she was presumptively diagnosed with paraneoplastic DM secondary to invasive ductal carcinoma of the left breast and treated with 20 mg prednisone daily. Her skin manifestations persisted and she subsequently developed upper extremity weakness. Her persistent and slowly progressing symptoms despite aggressive treatments prompted our decision to proceed with surgical intervention in hopes of preventing progression of her musculoskeletal manifestations. The patient underwent an uneventful left lumpectomy with left sentinel lymph node biopsy. Surgical pathology demonstrated a stage 2 poorly differentiated invasive ductal carcinoma (pT2N0). Postoperatively, she experienced rapid improvement in both cutaneous and musculoskeletal manifestations with visible clearing of her rash and return of her strength. There was also a concomitant decline in laboratory values (Table 1). She subsequently underwent adjuvant chemotherapy and radiation therapy. She was maintained on prednisone with excellent symptomatic control. On her 6-month visit, we noted resolution of most of her rash, periorbital edema as well as myalgias and muscle weakness (Fig. 6).
Laboratory values prior to the surgery, 3 weeks postoperatively and 6 weeks postoperatively.
| . | Preop . | 3 weeks postop . | 6 weeks postop . |
|---|---|---|---|
| White blood cell (×109) | 9.3 | 12.6 | 7.8 |
| Creatine kinase (U/l) | 2183 | 302 | 160 |
| Aldolase (U/l) | 17.4 | – | 5.3 |
| Myoglobin | 247 | – | – |
| AST (U/l) | 117 | 77 | 44 |
| ALT (U/l) | 53 | 91 | 30 |
| . | Preop . | 3 weeks postop . | 6 weeks postop . |
|---|---|---|---|
| White blood cell (×109) | 9.3 | 12.6 | 7.8 |
| Creatine kinase (U/l) | 2183 | 302 | 160 |
| Aldolase (U/l) | 17.4 | – | 5.3 |
| Myoglobin | 247 | – | – |
| AST (U/l) | 117 | 77 | 44 |
| ALT (U/l) | 53 | 91 | 30 |
Laboratory values prior to the surgery, 3 weeks postoperatively and 6 weeks postoperatively.
| . | Preop . | 3 weeks postop . | 6 weeks postop . |
|---|---|---|---|
| White blood cell (×109) | 9.3 | 12.6 | 7.8 |
| Creatine kinase (U/l) | 2183 | 302 | 160 |
| Aldolase (U/l) | 17.4 | – | 5.3 |
| Myoglobin | 247 | – | – |
| AST (U/l) | 117 | 77 | 44 |
| ALT (U/l) | 53 | 91 | 30 |
| . | Preop . | 3 weeks postop . | 6 weeks postop . |
|---|---|---|---|
| White blood cell (×109) | 9.3 | 12.6 | 7.8 |
| Creatine kinase (U/l) | 2183 | 302 | 160 |
| Aldolase (U/l) | 17.4 | – | 5.3 |
| Myoglobin | 247 | – | – |
| AST (U/l) | 117 | 77 | 44 |
| ALT (U/l) | 53 | 91 | 30 |
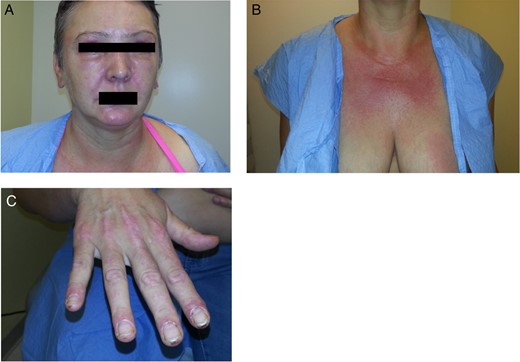
Image of patient with facial rash and periorbital edema that initially developed during her workup (A). Images of the worsening rash on her chest and hands that initially developed during her workup (B and C).
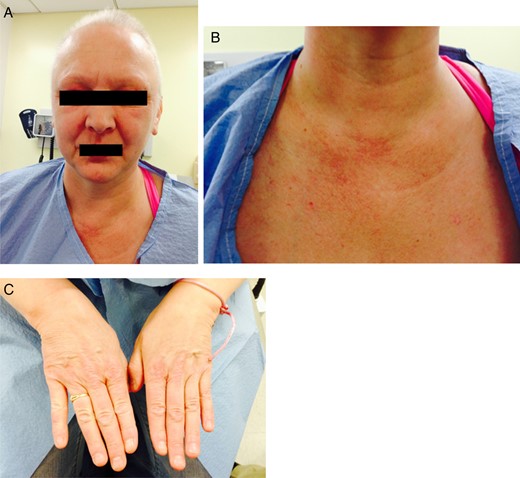
Images of patient at her 6-month follow-up visit. Note complete resolution of facial rash and periorbital edema and rash on her hand. There is almost complete resolution of the rash on her chest.
DISCUSSION
DM typically presents with progressive, symmetrical, proximal muscle weakness and characteristic skin lesions such as helitrope rash, Gottron's papules, Gottron's sign, the V-sign and shawl sign [5]. Additional cutaneous manifestations that have become more commonly recognized include vasculopathic changes (i.e. telangiectasias and livedo reticularis), cuticular overgrowth (‘mechanic's hands’) and poikiloderma. Initial presentation follows a bimodal distribution between ages 5–15 and 45–64 and tends to progress over a 3- to 6-month period before the patient will seek medical attention. Contrary to this typical presentation, the above patient experienced rapid progression of symptoms over a much shorter period and predominately reported skin manifestations with subsequent rapid development of muscular deterioration.
The diagnosis and classification of DM commonly follows the criteria set forth by Bohan and Peter in 1975 (Table 2) [1, 2]. Initial evaluation for suspected DM should include such investigations as serum creatine kinase, aldolase, aspartate aminotransferase, alanine aminotransferase and/or lactate dehydrogenase [5]. Serum creatine kinase is the most sensitive muscle enzyme in the acute phase of the disease as it is released in the serum during muscle damage. Serum inflammatory markers (e.g. erythrocyte sedimentation rate and C-reactive protein) may also be elevated during the active phase of the disease [5]. Our patient's laboratories were elevated preoperatively and declined rapidly. This decline was associated with the concurrent improvement in her symptoms.
| Individual criteria |
|
| Diagnostic criteria |
|
| Individual criteria |
|
| Diagnostic criteria |
|
| Individual criteria |
|
| Diagnostic criteria |
|
| Individual criteria |
|
| Diagnostic criteria |
|
Previous studies have shown that up to 30% of DM patients had underlying malignancies [6, 7]. Malignancies in DM patients have been found to be highest in those over 45 years of age, commonly diagnosed within the first year of initial presentation, and most strongly associated with lung, ovarian, gastric, pancreatic and colorectal origin [4]. Malignancy can precede, occur concurrently or develop after the diagnosis of DM. In our patient, the symptoms of DM occurred 2 weeks following her diagnosis of breast cancer.
The goals of therapy in patients with DM are to improve the patient's ability to carry out activities of daily living by increasing muscle strength and improving myalgias as well as treating the extramuscular manifestations (including rash and arthralgias). Systemic corticosteroids remain the mainstay medication in the treatment of DM [8]. There has been report of variable influence of the treatment of the underlying malignancy on the clinical course of the DM [9, 10]. In our patient, the complete surgical excision of the underlying breast cancer resulted in dramatic improvement in both her cutaneous manifestations and her musculosckeletal symptoms.
Given the high frequency of malignancy in patients with a diagnosis of DM, any woman over the age of 45 with newly diagnosed DM should prompt a thorough physical examination, including a breast examination, as well as further workup for other malignancies (e.g. ovarian or colorectal cancer). Early diagnosis of DM as a paraneoplastic syndrome allows for initiation of systemic therapy for symptom control and also treatment of the underlying malignancy itself, which may aid in control of the DM symptoms.
CONFLICT OF INTEREST STATEMENT
None declared.



